3370
Radiomics for noninvasive prediction of HCC immunophenotyping1BioMedical Engineering and Imaging Institute (BMEII), Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Diagnostic, Molecular and Interventional Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Pathology, Molecular and Cell Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Recanati/Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 5The Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 6Precision Immunology Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
The reported rate of intrahepatic recurrence of hepatocellular carcinoma (HCC) after resection is high (up to 50%), even with negative surgical margins, which is postulated to be due to micrometastases. Immunotherapy present new possibilities in cancer treatment with encouraging results in HCC. Selection of patients for immunotherapy may be based on background immune features, thus imaging features associated with immunophenotype may aid in patient selection. Specifically, radiomics features exhibited a fair to excellent diagnostic performance for differentiating tumors with high vs. low/moderate tumor infiltrating lymphocytes (TILs) and presence vs absence of tertiary lymphoid structure (TLS) with AUC range of 0.70-0.95.
Purpose
To assess the value of MRI radiomics quantification for prediction of HCC immune features at histopathology in a preliminary study.Background
Hepatocellular (HCC) is a leading cause of cancer-related deaths worldwide1, with increasing mortality in the USA2. Despite advances in therapy, the prognosis of HCC remains often poor due to high recurrence rates and detection at advanced stages3-4. Moreover, the introduction of biologic drugs including immune check-point inhibitors has revolutionized the HCC treatment approach. However, only a portion of HCC tumors respond to immunotherapy. The knowledge of tumor immune status could determine which patients are more likely to respond5-6. Prediction of HCC tumor biology, including histopathologic characteristics with imaging are unmet needs. The objective of our study is to assess the value of quantitative radiomics quantification in the prediction of HCC immunophenotypes.Methods
We quantified MRI radiomics features in a retrospective study of 39 patients (30M/9F, mean age 59y) with untreated HCCs who underwent hepatic resection within 3 months of gadoxetate MRI. A radiologist performed quantitative MRI analysis by segmenting the index HCC lesion on axial T2WI, DWI/ADC, post-contrast T1WI at the arterial (AP), portal venous (PVP), transitional (TP) and hepatobiliary phases post gadoxetate injection (HBP at 20min). Up to a total of 756 radiomics features were extracted, consisting of shape, 1st order (histogram), and 2nd order (texture) features. Histopathologic evaluation of representative H&E slides from the resected HCCs was retrospectively performed by a pathologist who assessed tumor grade, grade of tumor infiltrating lymphocytes (TILs, as low/moderate/high) and tertiary lymphoid structures (TLS, present/absent). The value of radiomics features for prediction of lymphocyte-rich tumors (TILs high grade) and tumors with TLS was assessed using a Mann Whitney U test and ROC analysis.Results
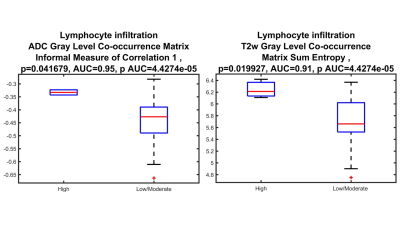
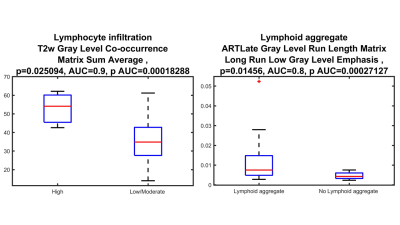
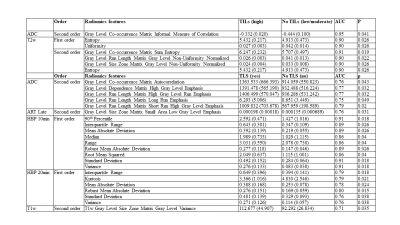
40 HCC lesions were evaluated (mean size 3.7 cm, range 1.3-14 cm). Pathological evaluation showed that most lesions (28/40=70%) were moderately or poorly differentiated. Immunophenotyping showed high TILs grade in 3 (7.5 %) and low/moderate TILs in 37 (92.5 %) tumors. Most tumors (27/40 = 67.5%) demonstrated TLS. Figure 1 represents the results of T2WI anatomical images at different phases along with pathology H&E. Radiomics features (shape, 1st and 2nd order) on ADC, T2WI, T1WI AP and HBP showed excellent diagnostic performance for distinguishing tumors with high vs. low/moderate TILs (AUC range: 0.70-0.95, p < 0.05) and between those with TLS vs no TLS (AUC range 0.70-0.91, p < 0.05). The highest AUCs (0.95 and 0.91) were observed for two 2nd order features on ADC (Gray Level Co-occurrence Matrix Informal Measure of Correlation) and T2WI (Gray Level Co-occurrence Matrix Sum Entropy) for identification of tumors with high grade TILs (Figure 2, 3 & Table 1).Discussion
This preliminary data shows the potential of radiomics feature to predict HCC immunophenotypes. Radiomics features that are associated with immunotherapy response may potentially aid in patient selection for immunotherapy. Study of radiomics features is rapidly growing for medical image analysis to aid the diagnosis, prognosis, patient’s management and prediction of treatment response within clinical decision-making system7. Radiomics features are sensitive to several factors, such as reconstruction settings8-9, tumor delineation10, scanning protocol11-12, different scanners13 and various noise source. We are waiting to have complete pathological data for ongoing prospective study and MICSSS for both to check whether MRI radiomics features could be noninvasive predictors of HCC immunophenotyping. Our study had several limitations such as retrospective study and a small cohort. In spite of the involvement of an experienced radiologist who identified lesions accurately, an exact geometric match could not be completely achieved by drawing freehand ROIs on tumorConclusion
Our preliminary study shows the potential of MRI radiomics features in predicting HCC immunophenotype, which may help predict response to immunotherapy. A validation cohort is required to confirm these findings.Acknowledgements
No acknowledgement found.References
1. Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
2. World Health Organization. Liver Factsheet. Globocan https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (2018).
3. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S (2015) Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 261:947–955
4. El-Khoueiry AB, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492–2502
5. Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563-67.
6. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568-71.
7. Kumar V, et al. Radiomics: The process and the challenges. Magn. Reson. Imaging 30, 1234–1248. https ://doi.org/10.1016/j. mri.2012.06.010 (2012).
8. Yan J, et al. Impact of image reconstruction settings on texture features in 18F-FDG PET. J. Nucl. Med. 56, 1667–1673. https: //doi. org/10.2967/jnume d.115.15692 7 (2015).
9. Galavis PE, Hollensen C, Jallow N, Paliwal B, & Jeraj R, Variability of textural features in FDG PET images due to different acquisition modes and reconstruction parameters. Acta Oncol. 49, 1012–1016. https ://doi.org/10.3109/02841 86X.2010.49843 7 (2010).
10. Veeraraghavan H, et al. Appearance constrained semi-automatic segmentation from DCE-MRI is reproducible and feasible for breast cancer radiomics: A feasibility study. Sci. Rep. 8, 4838. https ://doi.org/10.1038/s4159 8-018-22980 -9 (2018).
11. Ger RB, et al. Quantitative image feature variability amongst CT scanners with a controlled scan protocol. Proc. Spie https: //doi.org/10.1117/12.22937 01 (2018).
12. Saha A, Yu XZ, Sahoo D, & Mazurowski MA, Effects of MRI scanner parameters on breast cancer radiomics. Expert Syst. Appl. 87, 384–391. https ://doi.org/10.1016/j.eswa.2017.06.029 (2017).
13. Chirra P, et al. Empirical evaluation of cross-site reproducibility in radiomic features for characterizing prostate MRI. Proc. Spie https ://doi.org/10.1117/12.22939 92 (2018).
Figures
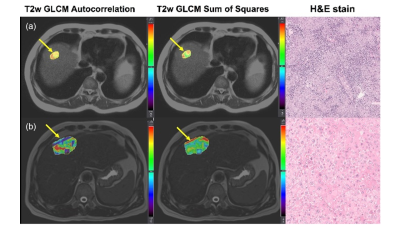
Figure 1: (a) Top row: 65-year-old male patient with HCC with high lymphocyte infiltration. Examples of T2WI anatomical images, and Gray Level Co-occurrence Matrix (GLCM) Autocorrelation and Sum of Squares radiomics features maps on T2WI, along with pathology H&E staining.(b) Bottom row: 55-year-old male patient with HCC with low lymphocyte infiltration. mean HCC T2WI GLCM Autocorrelation=1257.6, T2-w GLCM Sum of Squares=155.9; and b) mean HCC T2-w GLCM Autocorrelation=300.9, T2WI GLCM Sum of Squares=44.6. Tumor locations on MRI are highlighted with yellow arrows.