3363
Unique Alterations in the Pattern of Iron Deposition in Deep Gray Matter Relative to White Matter Microstructure in Mild Traumatic Brain Injury
Sohae Chung1, Els Fieremans1, Dmitry S. Novikov1, Prin X. Amorapanth2, Joseph F. Rath2, Steven R. Flanagan2, and Yvonne W. Lui1
1Department of Radiology, NYU Grossman School of Medicine, New York, NY, United States, 2Department of Rehabilitation Medicine, NYU Grossman School of Medicine, New York, NY, United States
1Department of Radiology, NYU Grossman School of Medicine, New York, NY, United States, 2Department of Rehabilitation Medicine, NYU Grossman School of Medicine, New York, NY, United States
Synopsis
Primary and secondary injury are both believed to play important roles in the pathogenesis of disease after mild traumatic brain injury (MTBI). Here we investigate the relationships between white matter microstructure and deep gray matter iron deposition after MTBI, which may shed light on primary WM injuries and potential secondary changes in brain iron. Our results show different patterns of correlation between deep gray matter iron content as measured by QSM and WM microstructure as measured by diffusion MRI in MTBI compared with normal controls.
Introduction
Mild traumatic brain injury (MTBI) is a major public health problem. Some MTBI patients recover quickly from a symptomatic perspective, however, a substantial number of MTBI patients suffer from persistent symptoms. In addition to primary injury, secondary effects are believed to contribute importantly to pathogenesis of persistent symptoms and longer-term problems. In particular, iron mishandling in the deep gray matter (GM) after injury has been implicated,1 yet the mechanisms of abnormal iron deposition are unclear. Some studies suggest that altered iron concentration could stem from white matter (WM) injury, interrupting WM pathways and triggering changes in iron content.2-3 Here we investigate the relationships between deep GM iron and WM microstructure in major WM regions in MTBI patients. Iron deposition was assessed via quantitative susceptibility mapping (QSM),4 sensitive to brain tissue iron content, and WM microstructure was assessed via multi-shell diffusion MRI, including DTI, DKI and compartment-specific WM tract integrity (WMTI)5 metrics.Methods
We studied 21 MTBI patients (36 ± 13 years old) within a month of injury and 41 normal controls (NC) (34 ± 10 years old). MRI was performed on 3T MR scanners (Skyra/Prisma, Siemens) using a 3D MGRE sequence (FOV = 220x170x75mm3, 1.25 mm-isotropic resolution, TR = 92ms, 20 TEs = 1.90:2.32:45.98ms). QSM was generated by using the MEDI toolbox6. Segmentation of the deep GM (caudate, putamen, pallidum, thalamus) was obtained by FreeSurfer and manual correction performed if needed.For WM, diffusion imaging was performed with 5 b-values (0.25, 1, 1.5, 2, 2.5 ms/µm2) along with a total of 137 diffusion-encoding-directions using multiband (factor of 2) (FOV = 220×220mm2, matrix = 88×88, 2.5 mm-isotropic resolution, slices = 56, TR/TE = 4.9s/95ms, and GRAPPA factor=2). We calculated 11 diffusion parametric maps of DTI (fractional anisotropy [FA], mean/axial/radial diffusivity [MD/AD/RD]), DKI (mean/axial/radial kurtosis [MK/AK/RK]), and WMTI metrics (axonal water fraction [AWF], intra-axonal diffusivity [Daxon], extra-axonal axial and radial diffusivities [De,par and De,perp]). Sixteen bilateral WM regions-of-interest (ROIs) were identified from the JHU ICBM-DTI-81 WM atlas7, including corpus callosum (genu/body/splenium), anterior/posterior/retrolenticular limb of internal capsule (aIC/pIC/rIC), inferior/superior cerebellar peduncle (ICP/SCP), cerebral peduncle (CP), anterior/posterior/superior corona radiata (aCR/pCR/sCR), posterior thalamic radiation (pTR), external capsule (EC), superior longitudinal fasciculus (SLF) and fronto-occipital fasciculus (FOF). Averaged diffusion values were calculated across the voxels of the WM skeleton within each ROI.
Inter-scanner harmonization of diffusion parametric maps was performed using ComBat8 pipeline. Partial correlation analysis adjusted for age and sex was performed between deep GM QSM and diffusion parameters in WM ROIs for each group.
Results
Significant correlation patterns are present between deep GM iron content and WM microstructure in both healthy controls and MTBI groups though the pattern of correlation differed between groups (Fig. 1A-B). Correlation coefficients (R) with p < 0.05 in each group are summarized in Fig. 1C. Briefly, correlation coefficients are ranged from -0.49 to -0.32 and from 0.35 to 0.55 in NC, and ranged from -0.66 to -0.46 and from 0.46 to 0.51 in MTBI.Discussion
It appears that there are different relationships between deep GM iron as measured by QSM and WM microstructure as measured by diffusion MRI between healthy controls and MTBI subjects. In healthy controls, the greatest number of correlations were present involving pallidal iron; whereas in MTBI subjects, correlations involving putaminal iron were most common. There were no or few correlations involving thalamic iron in controls and MTBI groups, respectively. Among 11 diffusion metrics, correlations involving AK were seen most frequently and no correlations were present involving the diffusivity metrics MD, AD and RD metrics. We find significant correlations to be present only involving DKI and WMTI metrics and these have been shown in previous works to be more sensitive to microstructural changes in MTBI.Conclusion
This work ties together two major areas of research interest in MTBI: abnormal iron and WM injury. Our work identifies novel patterns of altered relationships between deep GM iron content and WM microstructure in MTBI. These patterns may shed new and needed light on the relationship between primary and secondary injuries in MTBI.Acknowledgements
This work is supported in part by NIH R01 NS119767-01A1, R01 NS039135-11, R21 NS090349, R56 NS119767, DoD PT190013 and the Leon Lowenstein Foundation. This work is also performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net), a NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
- Nisenbaum et al. The presence and role of iron in mild traumatic brain injury: an imaging perspective. J Neurotrauma. 2014;31:301-307.
- Williams R et al. Pathogenic implications of iron accumulation in multiple sclerosis. J Neurochem. 2012;120:7-25.
- Bergsland N et al. White matter tract injury is associated with deep gray matter iron deposition in multiple sclerosis. J Neuroimaging. 2017;27:107-113.
- Liu C et al. Susceptibility-weighted imaging and quantitative susceptibility mapping in the brain. JMRI. 2015;42:23–41.
- Fieremans, E., et al. White matter characterization with diffusional kurtosis imaging. Neuroimage 58:177-188, 2011.
- Liu T et al. Accuracy of the morphology enabled dipole inversion (MEDI) algorithm for quantitative susceptibility mapping in MRI. IEEE TMI. 2012;31:816-24.
- Mori S et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570-582.
- Fortin J et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149-170.
- Ward RJ et al. The role of iron in brain ageing and neurogenerative disorders. Lancet Neurol. 2014;13:1045-1060.
Figures
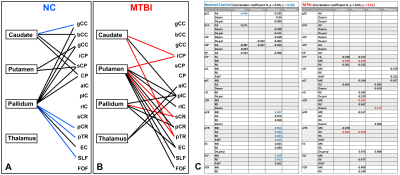
Figure 1. Different
correlation patterns between the deep gray matter iron content and WM microstructure
in (A) NC and (B) MTBI. The lines present connections that have at least one
significant correlation among diffusion metrics (black, p<0.05; red/blue,
p<0.01). Correlation coefficients (R) with p < 0.05 are summarized in (C).
DOI: https://doi.org/10.58530/2022/3363