3361
Initial Clinical Experience with MAGNUS Ultra-High-Performance Gradient Coil for Diffusion Microstructure Imaging of Intracranial Pathology1Uniformed Services University, Bethesda, MD, United States, 2Walter Reed National Military Medical Center, Bethesda, MD, United States, 3GE Research, Niskayuna, NY, United States
Synopsis
The MAGNUS ultra-high-performance gradient coil delivers simultaneous 200 mT/m and 500 T/m/s performance on each axis, with higher PNS thresholds than whole-body gradient coils, which is particularly useful for diffusion microstructure imaging. Our initial clinical experience with the MAGNUS research scanner has successfully identified white matter abnormalities (? intra-axonal edema) using multi-shell DTI (bmax = 4000 s/mm2) and OGSE (fmax = 100 Hz) in an acute symptomatic mTBI subject. It has also successfully identified differences in intracellular volume fraction using multi-shell multi-frequency OGSE (bmax = 2000 s/mm2, fmax = 100 Hz) between a low-grade diffuse astrocytoma and a high-grade glioblastoma.
Introduction
MAGNUS is a head-only ultra-high-performance gradient coil, which can be retrofitted into a clinical whole-body 3.0T MRI scanner. Its efficiency is derived from increased separation (18.6 cm) between the primary gradient layer and the secondary shield gradient layer, as permitted by a head-only design (inner diameter = 42 cm). Using a 1 MVA gradient driver, this research gradient coil delivers simultaneous 200 mT/m and 500 T/m/s performance on each axis, with higher PNS thresholds than whole-body gradient coils, which is especially useful for diffusion microstructure imaging (Figure 1).1-2We present an initial clinical experience with MAGNUS for diffusion microstructure imaging in our first subjects with intracranial pathology.
Methods
Under ongoing IRB-approved protocols, one subject with acute mild traumatic brain injury (mTBI) and two subjects undergoing pre-biopsy evaluation for suspected brain tumors were scanned on MAGNUS with both conventional and research sequences, in addition to healthy controls.The subject with acute mTBI developed new headaches and difficulty with attention/sleep after a rear-end motor vehicle collision. On day 9 post-injury, she was enrolled for research imaging. Multi-shell diffusion tensor imaging (DTI) was performed using 125 directions distributed across b = 0, 500, 2000, 4000 s/mm2, with an isotropic resolution of 1.5 mm3. Oscillating gradient spin echo (OGSE) diffusion imaging was performed at 5 frequencies from 0 to 100 Hz, using b = 450 s/mm2 and 30 directions per frequency, with an isotropic resolution of 2.2 mm3.
Two subjects with suspected brain tumors were recruited for microstructure imaging during their pre-biopsy evaluation. In addition to the multi-shell DTI and single-shell OGSE (identical protocol to mTBI patient), an additional multi-shell multi-frequency OGSE was also performed to characterize tumor compartment composition changes based on a spherical cell model, with b-values up to 2000 s/mm2 and frequencies up to 100 Hz.
Results
In our acute symptomatic mTBI subject, conventional T1-, T2-, T2*-, and diffusion-weighted sequences were unremarkable both on a clinical 3.0T scanner and on the MAGNUS research scanner. In contrast, visible abnormalities were depicted on a parallel kurtosis map generated from the multi-shell DTI (bmax = 4000 s/mm2) and on a time dependence of parallel diffusivity map generated from the OGSE (fmax = 100 Hz). They revealed decreased parallel kurtosis and increased time dependence of parallel diffusivity in the left parietal white matter, without any corresponding abnormalities on apparent diffusion coefficient (ADC) or fractional anisotropy (FA) maps (Figure 2). Similar abnormalities of parallel kurtosis or time-dependent diffusivity were not seen in our healthy controls.In our tumor subjects, one had a non-enhancing left frontal lobe mass, later diagnosed as an IDH-mutant diffuse astrocytoma WHO grade 2 on biopsy. The other had a heterogeneously-enhancing splenium mass, later diagnosed as an IDH-wildtype glioblastoma WHO grade 4 on biopsy (a small region of interest was drawn over the solid portion that was targeted during stereotactic needle biopsy). The multi-shell multi-frequency OGSE data was analyzed with a two-compartment model in order to generate estimates of intracellular volume fraction and cell size (Figure 3). There was a significant difference in the estimates of intracellular volume fraction but not cell size between diffuse astrocytoma and glioblastoma (Figures 4-5).
Discussion
Diffusion microstructure imaging has been studied extensively as a potential method to detect or differentiate neuropathology beyond the limitations of conventional MRI techniques. While research studies of DTI have demonstrated white matter abnormalities (e.g. low FA) in a subset of mTBI patients, presumed to represent axonal injury, these analyses are often dependent on quantitative analysis at the group level and are less robust at the individual level.3-4 Detection of normal axonal anatomy and traumatic axonal injury on microstructure techniques like OGSE is limited by maximum gradient amplitude (Gmax) < 80 mT/m on clinical scanners.5 On MAGNUS Gmax 200 mT/m, advanced DTI (bmax = 4000 s/mm2, TE~45 ms) and OGSE (fmax = 100 Hz, TE~125 ms) in our first subject with acute mTBI were able to detect abnormalities on maps of parallel kurtosis and time-dependent diffusivity, which were apparent both visually and quantitatively, possibly reflecting intra-axonal edema (less restricted non-Gaussian diffusion).For brain tumors, advanced microstructure imaging may help noninvasively differentiate among different phenotypes/genotypes, which can overlap in appearance on conventional sequences.6 While ADC values reflect a mixture of tissue properties, including cell size (µm) and cell density (mm-3), OGSE may be able to differentiate these contributions by measuring signal variations with different diffusion times, although higher gradient performance is needed to be sensitive to signal at the smallest diffusion times and length scales.7 With MAGNUS gradients, we were able to apply multi-shell multi-frequency OGSE (bmax = 2000 s/mm2, fmax = 100 Hz) and identify differences in time-dependent diffusivity between a diffuse astrocytoma and a glioblastoma, utilizing higher oscillating frequencies and lower diffusion times than previously reported on clinical scanners.8 Cell size estimates from OGSE have been validated histologically with cell membrane staining in other research studies on breast and colon cancer.9-10
Conclusion
Our initial clinical experience with the MAGNUS research scanner for diffusion microstructure imaging has successfully identified white matter abnormalities (? intra-axonal edema) in acute mTBI and differences in intracellular volume fraction between a low-grade diffuse astrocytoma and a high-grade glioblastoma.Acknowledgements
The views expressed in this abstract are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences, Walter Reed National Military Medical Center, the Department of Defense, or the U.S. Government.References
1. Foo TKF, Tan ET, Vermilyea ME, et al. Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magn Reson Med. 2020 Jun;83(6):2356-2369.
2. Tan ET, Shih RY, Mitra J, et al. Oscillating diffusion-encoding with a high gradient-amplitude and high slew-rate head-only gradient for human brain imaging. Magn Reson Med. 2020 Aug;84(2):950-965.
3. Amyot F, Arciniegas DB, Brazaitis MP, et al. A Review of the Effectiveness of Neuroimaging Modalities for the Detection of Traumatic Brain Injury. J Neurotrauma. 2015 Nov 15;32(22):1693-721.
4. Hulkower MB, Poliak DB, Rosenbaum SB, et al. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol. 2013 Nov-Dec;34(11):2064-74.
5. Drobnjak I, Zhang H, Ianuş A, et al. PGSE, OGSE, and sensitivity to axon diameter in diffusion MRI: Insight from a simulation study. Magn Reson Med. 2016 Feb;75(2):688-700.
6. Smits M, van den Bent MJ. Imaging Correlates of Adult Glioma Genotypes. Radiology. 2017 Aug;284(2):316-331.
7. Jiang X, Li H, Xie J, et al. Quantification of cell size using temporal diffusion spectroscopy. Magn Reson Med. 2016 Mar;75(3):1076-85.
8. Maekawa T, Hori M, Murata K, et al. Differentiation of high-grade and low-grade intra-axial brain tumors by time-dependent diffusion MRI. Magn Reson Imaging. 2020 Oct;72:34-41.
9. Xu J, Jiang X, Li H, et al. Magnetic resonance imaging of mean cell size in human breast tumors. Magn Reson Med. 2020 Jun;83(6):2002-2014.
10. Jiang X, Li H, Xie J, et al. In vivo imaging of cancer cell size and cellularity using temporal diffusion spectroscopy. Magn Reson Med. 2017 Jul;78(1):156-164.
Figures
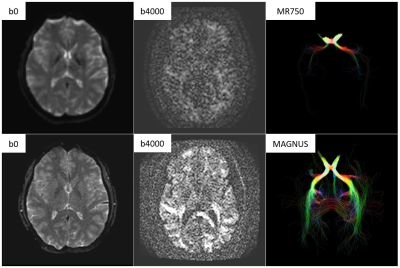
Benefits of MAGNUS ultra-high-performance gradients for microstructure imaging. The top row are b0, b4000, and tractography (seeded in the optic chiasm) images on a clinical 3.0T scanner (50 mT/m at 200 T/m/s). The bottom row are images on the MAGNUS scanner (200 mT/m at 500 T/m/s) in the same subject. Note decreased distortion next to the frontal sinuses on b0 as well as increased signal on b4000 and tractography images. Many of these benefits are related to decreased echo time (e.g. MAGNUS gradients can achieve TE < 50 ms at b = 10,000 s/mm2).
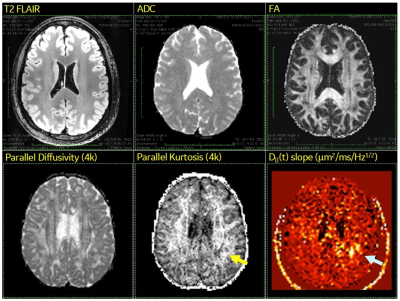
Possible findings of intra-axonal edema in acute symptomatic mTBI subject (9 days after motor vehicle accident). Routine structural and diffusion sequences (T2 FLAIR, ADC, FA) were visually unremarkable. In contrast, visible abnormalities were identified in the left parietal white matter (arrows) on a parallel kurtosis map generated from the multi-shell DTI (bmax = 4000 s/mm2) and on a time dependence of parallel diffusivity map generated from the OGSE (fmax = 100 Hz). The advanced DTI/OGSE sequences are made feasible by use of ultra-high-performance gradients.
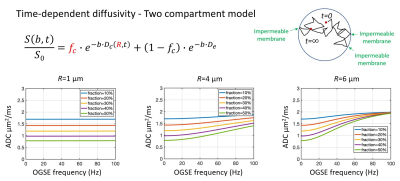
Two compartment model with signal contribution from both time-dependent diffusivity in the intracellular compartment (impermeable spheres) plus Gaussian diffusivity in the extracellular compartment for tumor microstructure imaging. Multi-shell multi-frequency OGSE was applied to estimate intracellular volume fraction (fc) and cell radius (R), which can be used to calculate cell density (3fc/4πR3). Bottom row are simulations of diffusivities versus frequency for varying combinations of cell sizes and intracellular volume fractions.
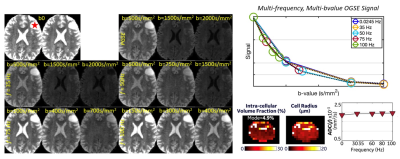
Multi-shell multi-frequency OGSE of left frontal brain tumor (red star) on the MAGNUS research scanner before biopsy, which confirmed an IDH-mutant diffuse astrocytoma WHO grade 2. The signal change with different b-values and frequencies was used to estimate intracellular volume fraction (fc) and cell radius (R) to be approximately 5% and 5 µm. In the lower right, a diffusivity versus frequency curve shows a relatively high y-intercept, which correlates with relatively high extracellular volume fraction (~95%).
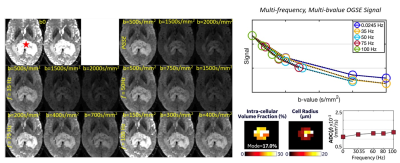
Multi-shell multi-frequency OGSE of a splenial brain tumor (red star) on the MAGNUS research scanner before biopsy, which confirmed an IDH-wildtype glioblastoma WHO grade 4. The OGSE signal change with different b-values and frequencies was used to estimate intracellular volume fraction (fc) and cell radius (R) to be approximately 17% and 4-5 µm. The lower right diffusivity versus frequency curve shows a relatively low y-intercept, which correlates with relatively low extracellular volume fraction (~83%).