3360
Associations between vascular health and brain stiffness1Mayo Clinic, Rochester, MN, United States
Synopsis
Vascular health is a predictor of cognitive outcomes, but the relationship between the two remains incompletely understood. Here we tested the hypothesis that brain stiffness is significantly associated with vascular health as assessed by white matter hyperintensity (WMH) load and the presence of systemic risk factors. Through voxel-wise mapping, WMH was associated with decreased stiffness in periventricular regions, while systemic risk factors were associated with widespread, lower-amplitude stiffness decreases. Examining the partial correlations between stiffness, WMH and vascular risk factors, stiffness was significantly associated with both measures, while WMH and vascular risk were not significantly correlated after controlling for stiffness.
Introduction
Vascular health is a significant predictor of poor neurological outcomes including stroke, hemorrhage, dementia and even death.1 Moreover, vascular health impacts cognition both directly and indirectly via Alzheimer’s disease (AD) pathology.2, 3 Nonetheless, the relationship between current measures of vascular health and clinical outcomes remains probabilistic, reflecting an incomplete understanding of the mechanisms that link the two, and suggesting that additional biomarkers may be beneficial in bridging the gap. Based on preliminary observations, the hypothesis of this study is that MR elastography-based biomarkers can provide a global indication of brain vascular health. To this end, we evaluated the association between brain stiffness as assessed by MR elastography with both cerebrovascular disease and systemic vascular health.Materials and methods
This IRB approved study included 75 participants who had undergone MRE exams and vascular health assessment after providing written informed consent. MRE data were acquired as part of previous studies of normal aging and AD.4, 5 Briefly, MRE data were acquired with a modified spin-echo EPI pulse sequence, 60 Hz vibration, and 3-mm isotropic sampling. Full data acquisition details can be found in the original manuscripts. Stiffness maps were estimated using a neural network inversion,6, 7 trained using data generated by a finite difference model of harmonic motion in a linear viscoelastic, inhomogeneous, and isotropic material. Cerebrovascular disease was assessed by T2 FLAIR imaging. T2 white matter hyperintensities (WMH) were segmented with a semi-automated in-house algorithm,8 and summarized as the log-transformed volume as a percentage of total intracranial volume. Systemic vascular health was assessed from the clinical record of each participant as a Cardiovascular and Metabolic Condition (CMC) score. This score was computed in each participant as the summation of the presence of hypertension, hyperlipidemia, cardiac arrhythmias, coronary artery disease, congestive heart failure, diabetes mellitus, and stroke.3Two statistical analyses were performed. First, voxel-wise modeling was performed to interrogate the topography of stiffness changes associated with each measure of vascular health. To this end, following spatial normalization of each participant’s stiffness map to template space,9 a linear model was fit to the data at each voxel with predictors including age, sex, WMH and CMC. Partial correlation analysis was also performed to assess the relationship between global brain stiffness (µ), WMH and CMC. P<0.05 was considered significant.
Results
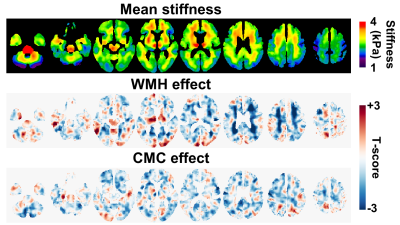
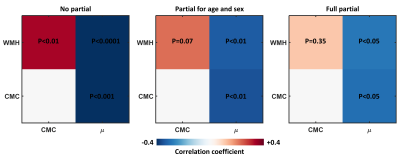
Figure 1 shows the mean stiffness map, as well as the estimated WMH and CMC effect maps. WMH was associated with decreased stiffness predominantly in periventricular regions, where hyperintensities are commonly located. On the other hand, the CMC effect was more widespread with increasing number of vascular conditions associated with decreased stiffness. The results of the partial correlation analysis are presented in Figure 2. Without any corrections, all pairwise correlations between variables of interest (µ, WMH, CMC) are statistically significant. When age and sex are fixed, the relationship between WMH and CMC is no longer significant. When a full partial correlation analysis is performed, µ is still significantly associated with WMH and CMC, but WMH and CMC are not significantly correlated. Taken together, the correlation analysis suggests that stiffness is sufficient to explain the relationship between WMH and CMC, but not vice versa.Conclusion
In summary, brain stiffness demonstrates non-overlapping sensitivity to both cerebrovascular disease and systemic vascular health. The mechanism for this decrease in stiffness with diminished vascular health will require further investigation, but it may reflect decreased perfusion, which is otherwise difficult to measure in white matter due to the low signal-to-noise ratio of arterial spin labeling in these regions.10, 11 The results provide strong motivation for further work to determine the extent to which MRE-based biomarkers for vascular health can improve the prediction of cognitive outcomes.Acknowledgements
This work was supported by the NIH grants EB001981 and EB027064.References
1. Debette S, Schilling S, Duperron M-G, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA neurology 2019;76:81-94.
2. Vemuri P, Lesnick TG, Knopman DS, et al. Amyloid, Vascular, and Resilience Pathways Associated with Cognitive Aging. Ann Neurol 2019;86:866-877.
3. Vemuri P, Lesnick TG, Przybelski SA, et al. Age, vascular health, and Alzheimer disease biomarkers in an elderly sample. Ann Neurol 2017;82:706-718.
4. Arani A, Murphy MC, Glaser KJ, et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage 2015;111:59-64.
5. Murphy MC, Jones DT, Jack CR, Jr., et al. Regional brain stiffness changes across the Alzheimer's disease spectrum. Neuroimage Clin 2016;10:283-290.
6. Murphy MC, Manduca A, Trzasko JD, Glaser KJ, Huston J, 3rd, Ehman RL. Artificial neural networks for stiffness estimation in magnetic resonance elastography. Magn Reson Med 2018;80:351-360.
7. Scott JM, Arani A, Manduca A, et al. Artificial neural networks for magnetic resonance elastography stiffness estimation in inhomogeneous materials. Med Image Anal 2020;63:101710.
8. Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, et al. White matter hyperintensities: relationship to amyloid and tau burden. Brain 2019;142:2483-2491.
9. Schwarz CG, Gunter JL, Ward CP, et al. [P2–415]: THE MAYO CLINIC ADULT LIFESPAN TEMPLATE: BETTER QUANTIFICATION ACROSS THE LIFESPAN. Alzheimer's & Dementia 2017;13:P792-P792.
10. van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magnetic Resonance in Medicine 2008;59:788-795.
11. van Osch MJP, Teeuwisse WM, van Walderveen MAA, Hendrikse J, Kies DA, van Buchem MA. Can arterial spin labeling detect white matter perfusion signal? Magnetic Resonance in Medicine 2009;62:165-173.
Figures