3358
Age-related differences in white matter tract length assessed via TRACULA1Rotman Research Institute, Baycrest, Toronto, ON, Canada
Synopsis
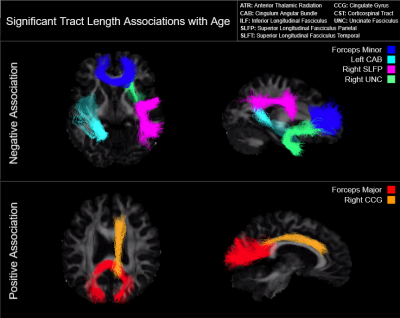
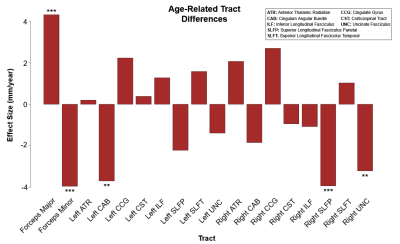
We examined differences in median tract length with age across eighteen major white matter tracts in 541 subjects of the Human Connectome Project in Aging. We observed a significant negative age effect on tract length in the forceps minor, left cingulum angular bundle, right superior longitudinal fasciculus parietal, and right uncinate fasciculus. Paradoxically, we also report a significant positive age effect on tract length in the forceps major and right cingulate gyrus. Restricted analyses suggest that these effects are driven by female subjects.
Introduction
Consistent changes in white matter structure and histology have been demonstrated in advancing age1,3,5,8,9. White matter volume decreases have been previously identified with normal aging, concomitant with decreases in fiber length observed in humans and animals using diffusion imaging and microscopy, respectively2,7. This loss in cumulative length can be attributed to the preferential culling of vulnerable axons, primarily in fibers with lower axonal diameter and less dense myelin sheaths9. Resulting whole brain changes are presumably due to a reduction in the total number of surviving fibers and increase in mean fiber diameter, rather than differences in tract shape or structure at the macro level. However, regional differences in this process are largely understudied. While white matter changes are documented across the brain, individual fiber tracts in the white matter exhibit varying degrees of vulnerability to age-related fiber culling5. In some instances, studies have shown both decreasing and increasing fiber length in specific major white matter bundles including the splenium and superior longitudinal fasciculus8, calling into question the assumption that overall decreases in fiber length correspond to regional decreases in tract length. This study examines the relationship between age, sex, and white matter tract length in healthy adults.Methods
Five hundred and thirty-five healthy adult subjects (300 female, aged 36-100) were drawn from the Human Connectome Project in Aging (HCP-A) dataset4. Subject data included whole-brain T1-weighted structural MRI measures and diffusion-weighted MRI (dMRI) collected using four matched Siemens Prisma 3T MRI scanners. Eighteen major white matter tracts were identified and reconstructed from subject dMRI data using the Tracts Constrained by Underlying Anatomy (TRACULA) tool in Freesurfer6,10 to produce per subject measures of individual tract length. Tract length was established by calculating the median length from fifteen hundred streamlines per tract generated by TRACULA. Samples outside the first and third quartiles in length were excluded as outliers. Median tract length measures were corrected for intracranial volume differences across subjects prior to analysis. Analysis was conducted using multivariate regression in R. Median tract lengths for the eighteen reconstructed tracts were regressed onto age, with separate analyses conducted for male, female, and combined sex data sets. False Detection Rate adjustment was applied to the resulting p-values to correct for multiple comparisons.Results
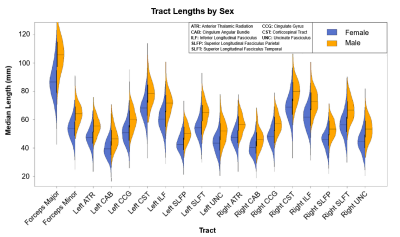
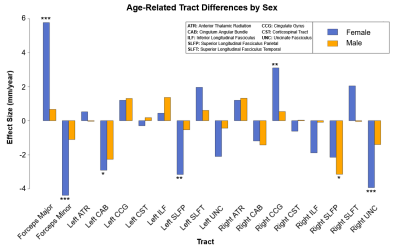
Multivariate regression found that age accounted for a significant amount of variance in tract length (F(18,516) = 10.05, p < .001, R2 = .26, R2Adjusted = .23). Tract-wise comparisons demonstrated both negative and positive associations between age and respective tract length (Figure 1). Negative relationships were identified in the forceps minor (p < .001), left cingulum angular bundle (p = .001), right superior longitudinal fasciculus parietal (p < .001), and right uncinate fasciculus (p = .005), indicating lower tract length in progressively older subjects. Conversely, the forceps major (p < .001) and right cingulate gyrus (p = .021) showed progressively greater length in older subjects (Figure 3). Restricting analysis by subject sex found that female subjects (F(18,281) = 9.54, p < .001, R2 = .38, R2Adjusted = .34) showed significant negative relationships between tract length and age in the forceps minor (p < .001), left cingulum angular bundle (p = .012), left superior longitudinal fasciculus parietal (p = .008), and right uncinate fasciculus (p < .001), as well as positive relationships between age and tract length in the forceps major (p < .001) and right cingulate gyrus (p = .008). Male subjects (F(18,216) = 3.19, p < .001, R2 = .21, R2Adjusted = .14) showed a single negative relationship between age and tract length in the right superior longitudinal fasciculus parietal (p = .034) tract (Figure 2, Figure 4).Discussion
Results confirm significant variations in white-matter tract lengths with age, however, tract changes were neither uniform between tracts nor uniform across sexes. These findings suggest that white matter tract length in female subjects changes considerably more with age than in male subjects. Whether these differences by sex correspond to differences in rates of white matter degeneration with age is unclear as female subjects demonstrated both decreases and increases in respective tract length with age, while male subjects demonstrated only decreases. Cumulatively, results indicated that whole brain white matter decreases in aging populations do not adequately model regional differences in age effects by tract. Without a clear model for the mechanism of these changes, the possibility remains that noted differences are non-predictive. Further research will be necessary to identify predictors of structural white matter changes with age, as well as the histological differences responsible for tract-wise and sex differences.Acknowledgements
Our thanks go out to the CIHR and NSERC for their support in funding this project.References
1. Baker, L. M., Laidlaw, D. H., Conturo, T. E., Hogan, J., Zhao, Y., Luo, X., Correia, S., Cabeen, R., Lane, E. M., Heaps, J. M., Bolzenius, J., Salminen, L. E., Akbudak, E., McMichael, A. R., Usher, C., Behrman, A., & Paul, R. H. (2014). White matter changes with age utilizing quantitative diffusion MRI. Neurology, 83(3), 247–252. https://doi.org/10.1212/WNL.0000000000000597
2. Baker, L.M., Laidlaw, D.H., Cabeen, R. et al. Cognitive reserve moderates the relationship between neuropsychological performance and white matter fiber bundle length in healthy older adults. Brain Imaging and Behavior 11, 632–639 (2017). https://doi.org/10.1007/s11682-016-9540-7
3. Bastin, M. E., Muñoz Maniega, S., Ferguson, K. J., Brown, L. J., Wardlaw, J. M., MacLullich, A. M., & Clayden, J. D. (2010). Quantifying the effects of normal ageing on white matter structure using unsupervised tract shape modelling. NeuroImage, 51(1), 1–10. https://doi.org/10.1016/j.neuroimage.2010.02.036
4. Bookheimer, S.Y., Salat, D.H., Terpstra, M., Ances, B.M., Barch, D.M., Buckner, R.L., Burgess, G.C., Curtiss, S.W., Diaz-Santos, M., Elam, J.S., Fischl, B., Greve, D.N., Hagy, H.A., Harms, M.P., Hatch, O.M., Hedden, T., Hodge, C., Japardi, K.C., Kuhn, T.P., Ly, T.K., Smith, S.M., Somerville, L.H., Uğurbil, K., van der Kouwe, A., Van Essen, D., Woods, R.P., Yacoub, E., 2019. The Lifespan Human Connectome Project in Aging: An overview. Neuroimage 185, 335–348.
5. Choy, S. W., Bagarinao, E., Watanabe, H., Ho, E., Maesawa, S., Mori, D., Hara, K., Kawabata, K., Yoneyama, N., Ohdake, R., Imai, K., Masuda, M., Yokoi, T., Ogura, A., Taoka, T., Koyama, S., Tanabe, H. C., Katsuno, M., Wakabayashi, T., Kuzuya, M., … Sobue, G. (2020). Changes in white matter fiber density and morphology across the adult lifespan: A cross-sectional fixel-based analysis. Human brain mapping, 41(12), 3198–3211. https://doi.org/10.1002/hbm.25008
6. Maffei, C., Lee, C., Planich, M., Ramprasad, M., Ravi, N., Trainor, D., Urban, Z., Kim, M., Jones, R., Henin, A., Hofmann, S., Pizzagalli, D., Auerbach, R., Gabrieli, J., Whitfield-Gabrieli, S., Greve, D., Haber, N., Yendiki, A. Using diffusion MRI data acquired with ultra-high gradients to improve tractography in routine-quality data. bioRxiv 2021.06.28.450265; doi: https://doi.org/10.1101/2021.06.28.450265
7. Marner, L., Nyengaard, J. R., Tang, Y., & Pakkenberg, B. (2003). Marked loss of myelinated nerve fibers in the human brain with age. The Journal of comparative neurology, 462(2), 144–152. https://doi.org/10.1002/cne.10714
8. Ouyang, Y., Cui, D., Yuan, Z., Liu, Z., Jiao, Q., Yin, T., & Qiu, J. (2021). Analysis of Age-Related White Matter Microstructures Based on Diffusion Tensor Imaging. Frontiers in aging neuroscience, 13, 664911. https://doi.org/10.3389/fnagi.2021.664911
9. Tang, Y., Nyengaard, J. R., Pakkenberg, B., & Gundersen, H. J. (1997). Age-induced white matter changes in the human brain: a stereological investigation. Neurobiology of aging, 18(6), 609–615. https://doi.org/10.1016/s0197-4580(97)00155-3
10. Yendiki, A., Panneck, P., Srinivasan, P., Stevens, A., Zöllei, L., Augustinack, J., Wang, R., Salat, D., Ehrlich, S., Behrens, T., Jbabdi, S., Gollub, R., & Fischl, B. (2011). Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in neuroinformatics, 5, 23. https://doi.org/10.3389/fninf.2011.00023
Figures