3357
Evaluation of the efficacy of a Deep Learning-based Reconstruction in the Connectomic Deep Brain Stimulation1Radiology / Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3GE Healthcare, Alberta, AB, Canada, 4GE Healthcare, New York, NY, United States, 5GE Healthcare, Menlo Park, CA, United States, 6Neurology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
The connectomic DBS approach, stimulation tractographically defined white mater pathways, has been successfully employed in functional neurosurgery, and it demonstrated the feasibility of clinical utility. However, this approach is limited in the clinical environment due to low SNR and various artifacts of DWI. The recent development of deep learning-based MR reconstruction allows us to improve SNR and reduce artifacts. This study evaluated the DL reconstruction method in the field of connectomic DBS using deterministic and probabilistic tractography. Tractography results from DL reconstruction show higher sensitivity for delineating WM pathways in specific DBS targets.
Introduction
The field of deep brain stimulation is currently experiencing a paradigm shift away from the focal effects of stimulation on the target anatomical structure toward its impact on activated brain networks[1]. This development is paralleled by a development of the “connectome” in the field of neuroimaging such as diffusion tractography with neural computational modeling that allows us to estimate WM pathways in each subject with given stimulation parameters (i.e., location, amplitude, pulse width)[2,3]. This connectomic DBS approach has been applied to subcallosal cingulate (SCC) DBS for depression, and it demonstrated the feasibility of clinical utility[4,5]. However, diffusion tractography has well-known pitfalls that limit the anatomical accuracy of the fiber pathways. These errors stem from image acquisition, estimation of orientation distribution function, and tracking streamline from voxel to voxel[6-10]. Numerous modern methods have been developed to address shortcomings in acquisition, modeling, and computation. Continuing efforts to improve image acquisition, a novel deep learning-based MR reconstruction method AIRTM Recon DL was recently proposed to improve image SNR, sharpness and reduce truncation artifacts[11]. In this study, we evaluated the efficacy of this DL reconstruction to improve the sensitivity of tractography in the connectomic DBS approach.Method
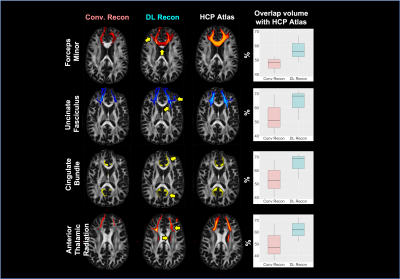
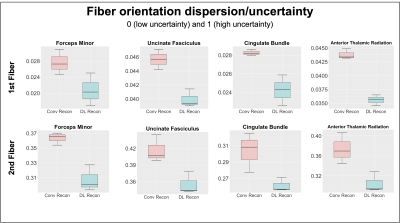
Participants and Data acquisition: Three DBS patients, including SCC for depression and anterior limb of internal capsule (ALIC) for OCD, were scanned under an IRB-approved protocol on a GE Architect 3T scanner (GE Healthcare, Waukesha, WI) using 48 channel head coil. High-resolution anatomical images were acquired using an Axial MPRAGE PROMO: 388 slices, 0.94x0.94x0.6mm3, TR=8.43ms, TE=3.18ms, TI=1100ms, FA=8. DWI data were collected using a SE-EPI sequence: 60 directions, 5 b0s, 80 slices, TR=17000ms, TE=74ms, slice thickness=2mm, in-plane resolution 2x2 mm2, in-plane acceleration=2. An opposite phase encoding b0 image was additionally collected to correct for the susceptibility-induced distortion. Data reconstruction: The same raw DWI data were reconstructed twice using conventional and DL reconstruction (Gibbs ringing removal and 75% denoising) . Processing: Data were preprocessed using a combination of tools from the FSL and MRTrix3 packages. The structural image was skull-striped and aligned to DWI native space image using a BBR registration tool, then registered to standard MNI space using a nonlinear registration. The same preprocessing pipeline was applied to both conventional and DL reconstructed DWI data. DWI data were first MP-PCA denoised[12], and head motion and susceptibility induced distortion was corrected[13]. Two different tractography algorithms (3dTrackID – deterministic and probtractx – probabilistic) were performed to evaluate WM bundles previously identified as therapeutic pathways for DBS (forceps minor, cingulum bundle, uncinate fasciculus, and anterior thalamic radiation). The XTRACT tool was used to automatically extract four WM bundles using probabilistic tractography[14]. Analysis: The results of deterministic tractography were qualitatively evaluated. For quantitative analysis, the fiber orientation dispersion (uncertainty) values, calculated from the Bayesian estimation of diffusion parameters (bedpostX)[15] between 0 and 1, were utilized because the dispersion (uncertainty) always reduces as SNR increases and artifacts reduce, regardless of the underlying tissue geometry (fanning or crossing) in the ball and stick model[16]. Then, the automatically extracted WM bundles from two reconstructed data were assessed by measuring the total overlap volume with the Human Connectome Project (HCP) atlase[17].Results
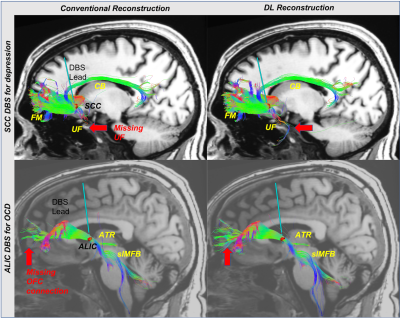
Results of deterministic tractography reveal that conventional reconstruction misses connections from the ALIC to Orbitofrontal in OCD and from the SCC to Amygdala (UF) in depression (Figure 1). Furthermore, the DL reconstruction shows higher tract density in the major WM bundles, including Cingulum and superolateral medial forebrain bundle. Automated probabilistic tractography shows consistent observations, missing parts of the tracts with conventional reconstruction and higher tract density with DL (Figure 2A). A better volume agreement with HCP atlas is observed with DL reconstruction, compared to conventional reconstruction. Lastly, DL reconstruction shows noticeably lower dispersion (uncertainty) in all four tracts than the conventional reconstruction (Figure 3) for both the first and the second fiber orientations, indicating effective noise and artifacts reduction with DL.Discussion
DL reconstruction shows higher sensitivity for delineating WM pathways related to DBS targets in qualitative and quantitative tractography analyses. Specifically, conventional reconstruction fails to identify connections between ALIC and OFC, previously reported in tract tracer and diffusion tractography studies[18]. Significantly this pathway is also associated with clinical improvement in OCD DBS. In SCC DBS, UF, which was previously reported as a critical therapeutic pathway in retrospective probabilistic tractography studies[4,5], was missed by conventional reconstruction. Although UF passing through the SCC region is difficult to isolate in deterministic tractography due to high turn angle between amygdala and prefrontal, artifact and noise around sinus, UF pathway were clearly observed with DL reconstruction. In addition, lower dispersion results in both first and second diffusion direction with DL reconstruction suggest better discrimination in crossing fibers. In sum, these findings support the feasibility of DL reconstruction in the clinical environment for connectomic DBS that required advanced DWI images. Scan times are mainly limited in the busy clinical MRI for archiving advanced DWI data with high SNR and high spatial or angular resolution. Therefore, DL reconstruction might help get useful connectome data for precise DBS targeting with a much shorter scan time.Conclusion
DL reconstruction demonstrated its feasibilities in connectomic DBS approach by reducing noise and artifacts.Acknowledgements
No acknowledgement found.References
1. Andreas Horn et al, Connectomic Deep Brain Stimulation 1st edition, Academic Press, 2021.
2. David C. Van Essen, Stephen M. Smith, Deanna M. Barch, Timothy E.J. Behrens, Essa Yacoub, Kamil Ugurbil, for the WU-Minn HCP Consortium. (2013). The WU-Minn Human Connectome Project: An overview. NeuroImage 80(2013):62-79.
3. Ashutosh Chaturvedi et al 2013 J. Neural Eng. 10 056023
4. Patricio Riva-Posse, Ki Sueng Choi, Paul E. Holtzheimer, Cameron C. McIntyre, Robert E. Gross, Ashutosh Chaturvedi, Andrea L. Crowell, Steven J. Garlow, Justin K. Rajendra, Helen S. Mayberg. Defining Critical White Matter Pathways Mediating Successful Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Depression, Biological Psychiatry 2014, 76(12): 963-969.
5. Riva-Posse, P., Choi, K., Holtzheimer, P. et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry 2018, 23:843–849.
6. Donahue CJ, Sotiropoulos SN, Jbabdi S, Hernandez-Fernandez M, Behrens TE, Dyrby TB, Coalson T, Kennedy H, Knoblauch K, Van Essen DC, Glasser MF, Using diffusion tractography to predict cortical connection strength and distance: a quantitative comparison with tracers in the monkey. J. Neurosci 2016, 36: 6758–6770.
7. Girard G, Whittingstall K, Deriche R, Descoteaux M, Towards quantitative connectivity analysis: reducing tractography biases. Neuroimage 2014, 98:266–278.
8. Maier-Hein KH, Neher PF, Houde JC, Cote MA, Garyfallidis E, Zhong J, Chamberland M, Yeh FC, Lin YC, Ji Q, Reddick WE, Glass JO, Chen DQ, Feng Y, Gao C, Wu Y, Ma J, Renjie H, Li Q, Westin CF, Deslauriers- Gauthier S, Gonzalez JOO, Paquette M, St-Jean S, Girard G, Rheault F, Sidhu J,Tax CMW, Guo F, Mesri HY, David S, Froeling M, Heemskerk AM, Leemans A, Bore A, Pinsard B, Bedetti C, Desrosiers M, Brambati S, Doyon J, Sarica A, Vasta R, Cerasa A, Quattrone A, Yeatman J, Khan AR, Hodges W, Alexander S, Romascano D, Barakovic M, Auria A, Esteban O, Lemkaddem A, Thiran JP, Cetingul HE, Odry BL, Mailhe B, Nadar MS, Pizzagalli F, Prasad G, Villalon-Reina JE, Galvis J, Thompson PM, Requejo FS, Laguna PL, Lacerda LM, Barrett R, Dell’Acqua F, Catani M, Petit L, Caruyer E, Daducci A, Dyrby TB, Holland-Letz T, Hilgetag CC, Stieltjes B, Descoteaux M, The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun 2017, 8: 1349.
9. Ning L, Laun F, Gur Y, DiBella EV, Deslauriers-Gauthier S, Megherbi T, Ghosh A, Zucchelli M, Menegaz G, Fick R, St-Jean S, Paquette M, Aranda R, Descoteaux M, Deriche R, O’Donnell L, Rathi Y, Sparse Reconstruction Challenge for diffusion MRI: validation on a physical phantom to determine which acquisition scheme and analysis method to use? Med. Image Anal 2015, 26:316–331.
10. Schilling KG, Janve V, Gao Y, Stepniewska I, Landman BA, Anderson AW, Histological validation of diffusion MRI fiber orientation distributions and dispersion. Neuroimage 2018, 165: 200– 221.
11. Lebel, R. M. (2020). Performance characterization of a novel deep learning-based MR image reconstruction pipeline. arXiv preprint arXiv:2008.06559.
12. L. Cordero-Grande, D. Christiaens, J. Hutter, A.N. Price, J.V. Hajnal Complex diffusion-weighted image estimation via matrix recovery under general noise models. NeuroImage 2019, 200: 391-404.
13. Jesper L. R. Andersson and Stamatios N. Sotiropoulos. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 2016, 125: 1063-1078.
14. Warrington S, Bryant K, Khrapitchev A, Sallet J, Charquero-Ballester M, Douaud G, Jbabdi S*, Mars R*, Sotiropoulos SN*. XTRACT - Standardised protocols for automated tractography and connectivity blueprints in the human and macaque brain. NeuroImage 2020: 217, 116923.
15. T.E.J. Behrens, M.W. Woolrich, M. Jenkinson, H. Johansen-Berg, R.G. Nunes, S. Clare, P.M. Matthews, J.M. Brady, and S.M. Smith. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 2003, 50(5):1077-1088.
16. S. Jbabdi, S.N. Sotiropoulos, A. Savio, M. Grana, T.E.J. Behrens. Model-based analysis of multishell diffusion MR data for tractography: How to get over fitting problems. Magn Reson Med, 2012,68(6):1846-1855.
17. Yeh, F. C., Panesar, S., Fernandes, D., Meola, A., Yoshino, M., Fernandez-Miranda, J. C., ... & Verstynen, T. Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage 2018, 178, 57-68.
18. Ziad Safadi, Giorgia Grisot, Saad Jbabdi, Timothy E. Behrens, Sarah R. Heilbronner, Nicole C.R. McLaughlin, Joe Mandeville, Amelia Versace, Mary L. Phillips, Julia F. Lehman, AnastasiaYendiki, Suzanne N. Haber, Functional Segmentation of the Anterior Limb of the Internal Capsule: Linking White Matter Abnormalities to Specific Connections, Journal of Neuroscience 2018, 38 (8) : 2106-2117
Figures