3355
Pain and the NeuroMatrix in the Brain: Feasibility and Safety Study of 3T DWI in a Chronic Low Back Pain Patient Treated with Spinal Cord Stimulation1Thomas Jefferson University, Philadelphia, PA, United States, 2Faculdades Pequeno Príncipe, Curitiba, Brazil
Synopsis
Spinal cord stimulation (SCS) in patients with failed back surgery syndrome aims to reduce neuropathic pain. However, minimal studies show a safe procedure using 3T diffusion weighted imaging (DWI) MRI for patients with SCS. Additionally, more studies looking at the neurological SCS treatment responses can lead to better optimization of SCS. It was our objective to safely develop a systematic procedure for 3T DWI MRI while also generating a map of the neural network connectivity in structures associated with pain. Long term application of this protocol aims to provide an objective assessment to SCS treatment.
Introduction
Many studies support spinal cord stimulation (SCS) as having the capacity to nullify chronic pain in patients with failed back surgery syndrome (FBSS)1-4. Although SCS is becoming popularized due to its pain relief, few studies have detailed a safe procedure for using 3T diffusion weighted imaging (DWI) MRI to observe regions associated with pain in SCS patients. To our knowledge, we created a protocol that is the first of its kind and will provide greater insight into the interconnectivity of white matter tracts for structures associated with pain. In particular, a pain neuromatrix of regions, such as those involved in the lateral and medial pain pathways, could be mapped5. These pathways in the brain serve to process the perception and discrimination of pain, respectively. By developing a safe systematic approach to 3T DWI, a neuromatrix can be developed and potentially used as a quantitative marker for the assessment of pain in SCS patients.Methods
One patient, a 55 year old male, underwent a series of advanced brain neuroimaging scans developed and optimized by our team. The stimulator was set to MR mode (off) prior to brain MRI. All scan parameters and MR equipment were adjusted to be in line with MRI guidelines for Medtronic neurostimulation systems (e.g., 2 W/kg whole body SAR, 3.2 W/kg head SAR, and up to 80% of the PNS limit). The MRI scans were performed using a 3.0T Siemens Prisma MR scanner (Siemens Healthcare, Erlangen, Germany) with a TR/TX head coil. DTI parameters used were number of directions=30, b-value=1000 s mm− 2, b0 images=2, voxel size=2 × 2 × 2mm3, slice thickness=2mm, TR=11.4 seconds, TE=85 ms, number of averages=1 and acquisition time=12:23 mins. To ensure safety and timelines of MRI scans, the patient was screened not only prior to scheduling an MRI visit but also before entering the MR scan room. All multimodal neuroimaging sequences were performed under safety policies and procedures. These sequences previously have been applied on healthy controls and quality of images have been confirmed.The spinal cord stimulation device is off label for scanning the brain under a 3T MRI. However, previous human and phantom studies reported no adverse effects scanning these patients for brain 3T MRI. The maximum SAR allowed by ICNIRP and FDA regulations is 4 W/kg whole body and 3 W/kg head only. Most MRI sequences have a SAR of ~0.5 W/kg total body. By using a transmit/receive head coil, the actual SAR at the level of the electrodes was even less.Diffusion processing:
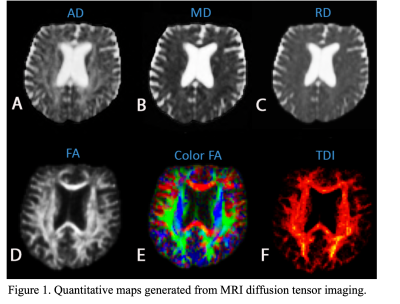
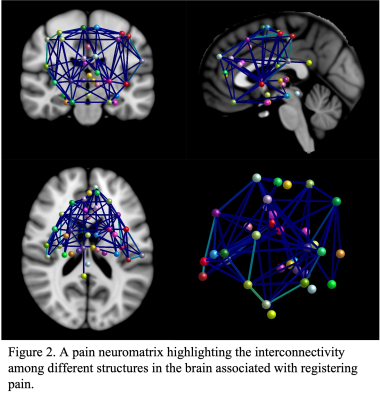
Initially, DWI MRI images were preprocessed to take into account motion correction, bias correction, denoising and then tensor fitting was applied to compute tensor maps resulting in fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD). Additionally, tract density map was created from whole brain deterministic tractography based on constrained spherical deconvolution (CSD) technique. Additionally, in-house software developed in Matlab (MathWorks, Natick, Massachusetts) was utilized to obtain a neuromatrix. The Neuromatrix contains Regions of interest (ROIs) associated in pain pathways including the S1: primary somatosensory cortex; M1: primary motor cortex; SMA: supplementary motor area; IN: insula region; MDT: mediodorsal thalamus; IT: intralaminar thalamus; VPL: ventral posterolateral nucleus; ACC: anterior cingulate cortex; PCC: posterior cingulate cortex; AMYG: amygdala; HIP: hippocampus; HT: hypothalamus; PAG: periaqueductal gray; DLPC: dorsolateral prefrontal cortex; MPF: medial prefrontal cortex; OC: orbitofrontal cortex; BG: basal ganglia and S2: secondary somatosensory cortex. These structures were extracted from both an AAL atlas and manual segmentation. We used the symmetric image normalization (SyN) method to register the NeuroMatrix (MNI space) to the diffusion space.
Results
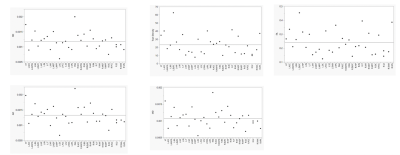
After processing the MRI images, different diffusion indices were obtained as shown in Figure 1. These quantitative indices include fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), mean diffusivity (MD) and track density imaging (TDI). Together, the data was compiled to create a pain neuromatrix (Figure 2) showing the interconnectivity in the white matter tracts. Lastly, the mean intensity was recorded from FA, AD, RD and MD to generate a quantitative representation of the pain neuromatrix (Figure 3). In regard to patient safety, no electrode displacement or hardware failure was observed during the MRI scanning sessions. Also SAR value (SAR=0.09W/Kg) was in line with safety guidelines. Profound device telemetry following MRI sessions showed no alterations in programmation.Discussion
Extrapolating the data presented will bolster the understanding of the connectivity within regions associated with pain, potentially serving as an objective assessment to SCS treatment. Moreover, the procedure was safely conducted, allowing for repeatability.Conclusion
DWI MRI is a non-invasive imaging procedure which can highlight changes in brain structure as it relates to treatment responses12-13. With this in mind, we developed a procedure to image and process regions in the brain associated with nociception to help further optimize SCS treatment. By incorporating MRI images and quantitative indices, a pain neuromatrix can serve as a quantitative biomarker or a complementary measure to report qualitative pain assessments. In summary, our data provides a starting point for future research into SCS treatment by providing a safe systematic approach to characterizing white matter tracts involved in ROIs associated with neuropathic pain following FBSS.Acknowledgements
N/AReferences
[1] Kumar K, Abbas M, Rizvi S. The use of spinal cord stimulation in pain management. Pain Manag. 2012;2(2):125-134. doi:10.2217/pmt.11.83
[2] Kumar K, Rizvi S. Historical and present state of neuromodulation in chronic pain. Curr Pain Headache Rep. 2014;18(1):387. doi:10.1007/s11916-013-0387-y[3] Kapural L, Peterson E, Provenzano DA, Staats P. Clinical Evidence for Spinal Cord Stimulation for Failed Back Surgery Syndrome (FBSS): Systematic Review. Spine (Phila Pa 1976). 2017;42 Suppl 14:S61-S66. doi:10.1097/BRS.0000000000002213
[4] Deer T, Slavin KV, Amirdelfan K, et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation. 2018;21(1):56-66. doi:10.1111/ner.12698
[5] Yen, Chen-Tung & Lu, Pen-Li. (2013). Thalamus and pain. Acta anaesthesiologica Taiwanica : official journal of the Taiwan Society of Anesthesiologists. 51. 73-80. 10.1016/j.aat.2013.06.011.
[6] Pahapill PA, Chen G, Arocho-Quinones EV, Nencka AS, Li SJ. Functional connectivity and structural analysis of trial spinal cord stimulation responders in failed back surgery syndrome. PLoS One. 2020 Feb 19;15(2):e0228306.
[7] Groote SD, et al. A Regions of Interest Voxel-Based Morphometry Study of the Human Brain During High-Frequency Spinal Cord Stimulation in Patients With Failed Back Surgery Syndrome. Pain Pract. 2020 May 29.
[8] De Groote S, Goudman L, Peeters R, Linderoth B, Vanschuerbeek P, Sunaert S, De Jaeger M, De Smedt A, Moens M. Magnetic Resonance Imaging Exploration of the Human Brain During 10kHz Spinal Cord Stimulation for Failed Back Surgery Syndrome: A Resting State Functional Magnetic Resonance Imaging Study. Neuromodulation. 2020 Jan;23(1):46-55.
[9] Deogaonkar M, Sharma M, Oluigbo C, Nielson DM, Yang X, Vera-Portocarrero L, Molnar GF, Abduljalil A, Sederberg PB, Knopp M, Rezai AR. Spinal Cord Stimulation (SCS) and Functional Magnetic Resonance Imaging (fMRI): Modulation of Cortical Connectivity with Therapeutic SCS. Neuromodulation. 2016 Feb;19(2):142-53.
[10] Moens M, Droogmans S, Spapen H, Smedt AD, Brouns R, Schuerbeek PV, Luypaert R, Poelaert J, Nuttin B. Effects of spinal cord stimulation on voxel-based brain morphometry in patients with failed back surgery syndrome. Clin Neurol Neurosurg. 2012 Feb;114(2):135-41.
[11] Moens M, Droogmans S, Spapen H, Smedt AD, Brouns R, Schuerbeek PV, Luypaert R, Poelaert J, Nuttin B. Spinal cord stimulation modulates cerebral function: an fMRI study. Neuroradiology. 2012 Dec;54(12):1399-407.
[12] Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A. Understanding the Physiopathology Behind Axial and Radial Diffusivity Changes-What Do We Know?. Front Neurol. 2018;9:92. Published 2018 Feb 27. doi:10.3389/fneur.2018.00092
[13] Tae WS, Ham BJ, Pyun SB, Kang SH, Kim BJ. Current Clinical Applications of Diffusion-Tensor Imaging in Neurological Disorders. J Clin Neurol. 2018;14(2):129-140. doi:10.3988/jcn.2018.14.2.129