3353
Patient-specific hyperdirect pathway activation in DBS for Parkinson's disease1Department of Neurosurgery, University Hospital Bern, Bern, Switzerland, 2ARTORG Center for Biomedical Engineering, University of Bern, Bern, Switzerland
Synopsis
Deep brain stimulation is an effective treatment in advanced Parkinson’s disease. The aim of the study was to determine activation thresholds for the hyperdirect pathway and corticospinal tract to control motor symptoms and avoid the appearance of side effects. Patient-specific whole brain tractograpy, DBS leads reconstruction, and generation of volumes of tissue activated were performed in 20 Parkinson patients. The activation threshold for the hyperdirect pathway was 60%, and for the corticospinal was 4%. For newly generated volumes of tissue activated, the average prediction error for the hyperdirect pathway was 1 mA and for the corticospinal tract was 1.5 mA.
INTRODUCTION
Deep Brain Stimulation (DBS) is an effective treatment in advanced Parkinson’s disease patients to control motor symptoms such as bradykinesia, rigidity, or tremor. DBS leads are implanted in the basal ganglia, which is connected to the cortex through, e.g., the direct, indirect and hyperdirect pathways. Recent studies suggest that DBS stimulation of the hyperdirect pathway plays an important role in the effects of the treatment1.The aim of the study was to determine an activation threshold of the hyperdirect pathway that results in the control of motor symptoms, and an activation threshold of the corticospinal tract to avoid the appearance of capsular side effects. The secondary aim was to predict the outcome of newly generated volumes of tissue activated using the obtained activation thresholds.
METHODS
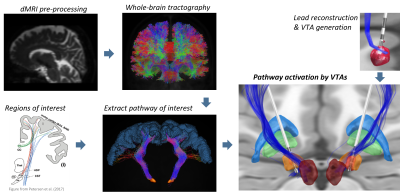
The study included 20 Parkinson’s disease subjects (9 female) undergoing bilateral DBS of the subthalamic nucleus. DBS patients were clinically assessed four to six months after the implantation with a monopolar review of omnidirectional and directional DBS (e.g., effects of rigidity, tremor, and bradykinesia) as previously reported2.Diffusion weighted MRI was used to perform whole-brain tractography in MRtrix3. Data pre-processing included denoising, Gibbs unringing and distortion and eddy-current correction. The filtering algorithm SIFT23 was applied to the whole-brain tractogram, assigning weights to the generated streamlines. The hyperdirect pathway and corticospinal tract were extracted from the whole-brain tractogram using cortical and subcortical regions of interest and regions of avoidance.
Lead reconstruction was performed in the Lead-DBS toolbox (v2.2). For each lead, volumes of tissue activated (VTAs) for every contact were created in the range of 0.5 – 8 mA in 0.5 steps. The VTAs from the postoperative assessment were used to estimate the activation of the hyperdirect pathway and the corticospinal tract. These VTAs were considered as “effect”, while the VTAs generated at 0.5 mA were considered as baseline for “no effect”. With the results of the pathway activation, we trained a logistic regression model to differentiate between “effect” and “no effect”, and we obtained the activation thresholds for both pathways. The activation thresholds were obtained as the weights' percentage of streamlines activated by the VTAs.
In a second step, we validated the pathway activation model using the generated VTAs for each contact. We excluded one lead at a time, and trained the logistic regression model with N-1 leads using the clinical stimulation VTAs. Then, we tested the model for the excluded lead. We obtained the model prediction for all VTAs in the range 1-8 mA and, for each contact, we selected the lowest stimulation amplitude resulting in full improvement (activation of the hyperdirect pathway) or in the appearance of capsular side effects (activation of the corticospinal tract). Finally, for each clinical VTA in the test lead, we compared the clinical stimulation amplitude with the model’s best prediction.
RESULTS
The logistic regression model resulted in an activation threshold of 60% for the hyperdirect pathway to obtain a full clinical effect and an activation threshold of 4% of the corticospinal tract for the appearance of capsular side effects (see figure 2). 10-fold cross-validation was used to assess the accuracy of the model. For the hyperdirect pathway, the average accuracy was 76.5 ± 0.08, and for the corticospinal tract 78.7 ± 0.06.We calculated the prediction error for the leave-one-lead out cross-validation as:
$$ Prediction\ error = predicted\ mA - clinical\ mA $$
where predicted mA corresponded to the stimulation amplitude predicted by the model for a given electrode contact and clinical mA was the stimulation amplitude recorded for this same contact during the clinical assessment.
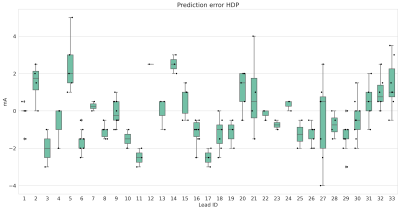
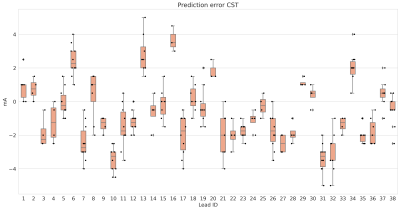
Figure 3 shows the absolute prediction error on average for all VTAs, and figures 4 and 5 show the prediction error for each lead individually.
DISCUSSION
We determined activation thresholds for the hyperdirect pathway and corticospinal tract on a group of 20 Parkinson's disease subjects. On average, the accuracy of the model to estimate the outcome of newly predicted VTAs was satisfactory. But on the individual lead analysis, there was more variability. For both the hyperdirect pathway and corticospianl tract, there was no clear tendency of overestimation or underestimation in the prediction of the stimulation amplitude. The stimulation amplitudes and clinical outcomes used for training the model corresponded to the clinical assessment performed 4 to 6 months after the surgery. Further studies containing chronic stimulation data, usually recorded one year after surgery, may improve the prediction for individual leads.CONCLUSION
Accurate activation models of the hyperdirect pathway and corticospinal tract will allow in the future a more precise and personalized targeting in DBS treatments for Parkinson’s disease to control motor symptoms and avoid the appearance of capsular side effects.Acknowledgements
Swiss National Science Foundation Project number 186142.References
1. Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science (80-. ). 324, 354–359 (2009).2. Nguyen, T. A. K. et al. Analysis of patient-specific stimulation with segmented leads in the subthalamic nucleus. PLoS One 14, e0217985 (2019).
3. Smith, R. E., Tournier, J. D., Calamante, F. & Connelly, A. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. Neuroimage 119, 338–351 (2015).
Figures
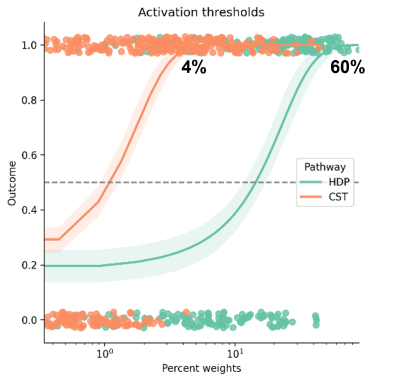
Activation thresholds for the hyperdirect pathway (HDP) and corticospinal tract (CST). Outcome = 0 corresponds to "no effect", and outcome = 1 corresponds to "effect".
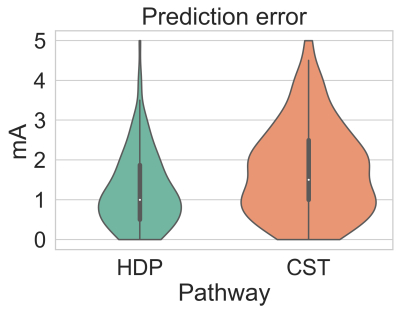
Average absolute prediction error for the hyperdirect pathway (HDP) and corticospinal tract (CST).