3351
Role of PET and ASL simultaneously acquired imaging biomarkers in differentiating progression from radionecrosis in glial brain tumors1CENIR, ICM, Paris, France, 2Pitié-Salpêtrière Hospital, APHP, Sorbonne universite, Paris, France, 3ICM, Paris, France, 4Pitié Salpêtrière, APHP, Paris, France
Synopsis
We aimed at investigating the methods based on coupling of of amino-acid metabolism (18F-DOPA-PET) measurements and cerebral blood flow (CBF) maps to evaluate the diagnostic performance of PET/MRI in glioma imaging. Tumor isocontour maps and T-maps were created using SPM and metabolic/perfusion abnormalities were evaluated with the asymmetry index z-score. SPM map analysis of significant-size clusters and semi-quantitative evaluation were performed and compared to gold standard diagnosis. Both the tumor isocontour maps and T-maps showed the highest specificity for ASL and sensitivity for 18F-DOPA analysis, allowing high diagnostic performance in differentiating between progression and radionecrosis particularly in high-grade treated gliomas.
INTRODUCTION
Surgery resection of adult brain glioma is often followed by adjuvant radiotherapy and/or chemotherapy1. Moreover, complete remission, when occurs, is usually time-limited and the risk of tumor recurrence is high, especially for high-grade glial lesions2. MRI is the modality of choice for the glial tumor follow-up. Whenever new or increased contrast enhancement is detected, it is particularly important to distinguish between treatment-related necrosis and a recurrent tumor. However, it often appears difficult to do this with conventional MRI3. Advanced MRI and, in particular, perfusion-weighted MRI (PWI) can be helpful to detect neoangiogenesis, related to tumor progression4. Dynamic susceptibility contrast (DSC)-PWI is a part of common tumor characterization and follow-up procedures. The alternatives to DSC-PWI in assessing cerebral blood flow (CBF) is ASL-PWI, being advantageous as it is less sensitive to post-therapeutic susceptibility artifacts5. 18F-DOPA (18F-fluoro-3,4-dihydroxy-L-phenylalanine)-PET is another imaging modality used to evaluate the post-therapeutic profile of brain tumors and is now part of the recommendations in the tumor follow-up6. However, in some patients, this method fails to differentiate between viable tumor tissue and necrosis. Combined PET/MR offers the opportunity to acquire biomarkers simultaneously, which could allow to increase the diagnostic accuracy.In the present work we aimed at studying, in a reproducible quantitative way, cerebral perfusion measured by ASL-MRI (rCBF) and glucose metabolism measured by 18F-DOPA-PET (rSUV) in post-therapeutic glioma of different grades to evaluate the potential of hybrid PET/MR in characterizing the tumor progression profile.
MATERIALS AND METHODS
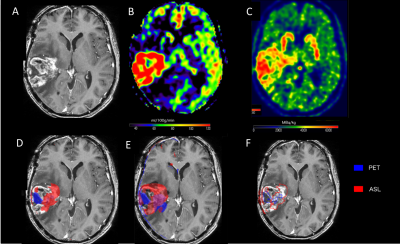
Between 2015 and 2017, patients followed for primary malignant brain tumors underwent FDOPA PET-MRI for the differential diagnosis between tumor progression and radionecrosis or pseudoprogression. The acquisitions were performed with a PET/MR system (SIGNA, GE Healthcare, Milwaukee, USA) 10 min after the injection of 2 MBq/kg of FDOPA. The MRI acquisition included Spin-echo (SE) 3D T1-weighted (T1-w) images without contrast and after injection of 0.2 ml/kg of Gadolinium (Gd)-DOTA (0.5 M Dotarem, Guerbet, Roissy, France), 3D FLAIR imaging, suseptibility weighted angiography (SWAN), diffusion weighted imaging (DWI) and pseudo-continuous arterial spin labeling (pCASL) and dynamic susceptibility-contrast (DSC) perfusion. Scanning time included a 20 min single-bed-position PET emission scan. PET images were reconstructed with an OSEM algorithm integrating TOF, PSF modeling and attenuation and scatter correction with 8 iterations and 28 subsets, a FOV of 300 x 300 mm2, and a voxel size of 1.17x1.17x2.78mm3.Data were preprocessed with statistical parametric mapping (SPM), including registration on T1-weighted images, spatial and intensity normalization, and tumor segmentation. As index tests, tumour isocontour maps of 18F-DOPA-PET and ASL T-maps were created and metabolic/perfusion abnormalities were evaluated with the asymmetry index z-score. SPM map analysis of significant-size clusters and semi-quantitative PET and ASL map evaluation were performed and compared to the gold-standard diagnosis. Lastly, ASL and PET topography of significant clusters was compared to that of the initial tumor. The results were compared with the gold standard being histology results or follow-up at more than 3 months.
Statistical analysis: The results of the analysis of each SPM map as well as of the CBF and PET readings were compared to the gold standard diagnosis. Sensitivity, specificity, PPV and NPV were calculated. The subgroup analyses for low- and high-grade and for WHO III and WHO IV tumors were performed and the subgroups were compared between them using Kruskal-Wallis non-parametric statistics.
RESULTS
Fifty-eight patients with treated unilateral gliomas who were included in the study constituted a group of 10 (17.2%) low-grade and 48 (82.8%) high-grade gliomas.Contrast enhancement was observed in 50 patients (86.2%), 16 cases corresponding to radionecrotic lesions (32%) and 34 cases corresponding to progression (68%:). 1
8F-DOPA-PET (rSUV) images showed significant hyper-metabolism 94.1% patients, including 3 patients (100%) with low-grade and 29 patients (93.5%) with high-grade gliomas. The overall sensitivity and specificity were 94.1% and 79.2%, respectively.
The tumor isocontour maps and T maps showed the highest specificity (100%) for ASL-rCBF analysis and highest sensitivity (94,1%) for rSUV analysis. The sensitivity of qualitative SPM ASL-rCBF maps was highest for high grade gliomas as compared to low-grade gliomas while there was no significant difference for 18F-DOPA-PET-rSUV analysis.
DISCUSSION AND CONCLUSION
In the present work, we propose a method of simultaneous analysis of ASL perfusion and 18F-DOPA-PET maps for the evaluation of glial tumour progression. Tumoural isocontour and T-map analyses has shown high diagnostic accuracy with the highest specificity for ASL-CBF analysis and the highest sensitivity for 18F-DOPA analysis, suggesting the complementarity of the markers. The complementarity seems to be particularly significant for the high-grade tumors, while ASL seems to have low input in the low-grade ones, related to the low neoangiogenesis in these lesions.Simultaneous analysis of PET/MR using the tumor isocontour and T-maps and the 18F-DOPA PET and ASL biomarkers allows improving diagnostic performance in differentiating between tumor progression and radio-necrosis especially in the high grade tumors (glioblastomas). This can help differentiate between tumour progression and pseudo-progression or radio-necrosis. This method can be promising for multicenter studies or biobank analyses where unbiased reproducible techniques are required.
Acknowledgements
No acknowledgement found.References
1. Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, DelattreJY. Primary brain tumours in adults. Lancet Lond Engl 2012, 379:1984–1996
2. Klobukowski L, Falkov A, Chelimo C, Fogh SE. A retrospective review of reirradiating patients’ recurrent high-grade gliomas. Clin Oncol 2018
3. Kumar AJ, Leeds NE, Fuller GN et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000. 217:377–384
4. Rossi A, Gandolfo C, Morana G, Severino M, Garrè ML, Cama A. New MR sequences (diffusion, perfusion, spectroscopy) inbrain tumours. Pediatr Radiol 2010. 40:999–1009
5. Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X. Arterial spin labeling perfusion of the brain: emerg-ing clinical applications. Radiology 2016. 281:337–356
6. Galldiks N, Stoffels G, Ruge MI et al. Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection ofmalignant progression in patients with low-grade glioma. J NuclMed 2013. 54:2046–2054
7. Brandes AA, Tosoni A, Spagnolli F et al. Disease progres-sion or pseudoprogression after concomitant radiochemotherapytreatment: pitfalls in neurooncology. Neuro Oncol 2008. 10:361–367
Figures