3350
Voxel-wise Comparison of Clinical 2D pCASL and DSC Perfusion of Intra-axial Brain Tumors1Radiology, UZ Leuven, Leuven, Belgium, 2Neuroradiology, UZ Leuven, Leuven, Belgium
Synopsis
Dynamical susceptibility contrast (DSC) and arterial spin labeling (ASL) are MRI techniques to quantify brain tissue perfusion. A voxel-wise analysis in 23 patients showed a moderate to strong linear relationship between tumoral cerebral blood flow measured by 2D echo planar imaging, pseudo-continuous ASL (pCASL) and leakage corrected, cerebral blood volume measurements by DSC. Additionally, mean and 90th percentile of tumor perfusion of all patients showed a strong linear relationship. In conclusion, 2D EPI pCASL is a viable alternative to DSC for perfusion measurements of brain tumors.
Introduction
Predicting brain tumor grade and differentiation remain challenging with conventional MRI and CT. Therefore, non-conventional MRI techniques, such as diffusion weighted imaging (DWI) and perfusion weighted imaging (PWI), are used to add functional information on brain tumors. Higher grade tumors and metastases often form new blood vessels in a process called neovascularization, increasing the tumoral blood flow and volume. With perfusion MRI, this process is visualized by quantifying the blood delivered to brain tissue.The most used PWI techniques in neuro-oncology are dynamic susceptibility contrast (DSC) and dynamic contrast enhancement (DCE). The major disadvantages of these techniques are the dependence on exogenous contrast administration and their non-repeatability. In contrast, arterial spin labeling (ASL) dynamically estimates blood delivery to brain tissue using magnetically labeled arterial blood protons as an endogenous tracer. The use of this technique in clinical practice is however less researched.
Since there is a plethora of different measurement techniques and analysis methods in PWI, a direct comparison of the advantages and disadvantages of different techniques remains difficult. We sought to make a direct voxel-wise comparison between our clinically acquired ASL and DSC sequences in patients with a newly diagnosed brain tumor.
Methods
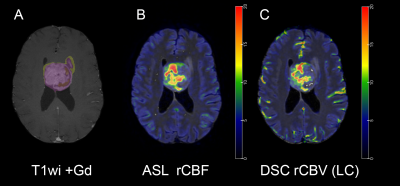
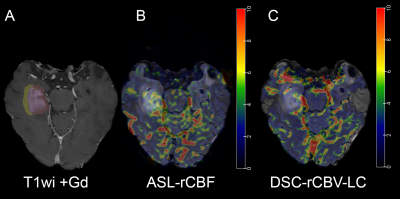
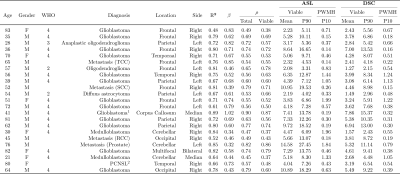
Twenty-three patients with a new, intra-axial, biopsy proven primary brain tumor or metastasis were scanned on a Philips Ingenia 3T between 01/12/2019 and 21/04/2021. Routine clinical sequences included T2-weighted imaging (T2wi), fluid-attenuation inversion recovery (FLAIR) imaging, DWI, susceptibility weighted imaging (SWI), and T1-weighted imaging pre (T1wi -Gd) and post Gadolinium (T1wi +Gd).The pseudo-continuous ASL (pCASL) protocol consisted of a two‐dimensional single‐shot echo planar imaging readout (1.8 seconds post label delay). Cerebral blood flow maps (ASL-CBF) were automatically generated on the scanner. The T2* gradient recalled echo DSC sequence was acquired with preload administration of Gadolinium. DSC post processing was performed in MATLAB to generate the leakage corrected, cerebral blood volume map (DSC-CBV-LC).
All images were skull stripped and coregistered using affine coregistration. Tumor segmentation was performed using a semi-automatic classification algorithm. Coregistration of the DSC perfusion was performed using the T2wi as intermediary to register the multiphasic DSC images. Grey matter probability maps were generated and subsequently smoothed to coregister the ASL-CBF map with masking to the tumor volume1. Perfusion maps of DSC and ASL were normalized to contralateral normal white matter, resulting in DSC-rCBV-LC and ASL-rCBF images (e.g. Figure 1 and 2).
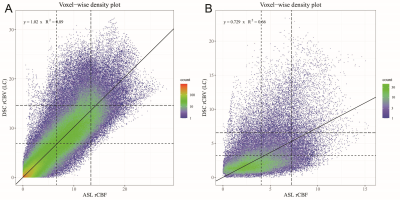
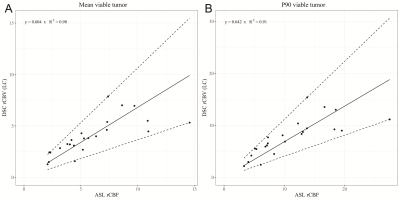
Voxel-wise analyses of the ASL-rCBF and DSC-rCBV-LC images per patient were performed using the Spearman correlation coefficient (ρ) and linear regression (coefficient of determination: R², and slope coefficient: β) with F-statistic. Subsequently, linear regression was performed on the mean values and p90-quantiles of perfusion measured by ASL and DSC of the pooled patient cohort.
Results
Results are shown in Table 1. All voxel-wise linear correlations between the individual ASL-rCBF and DSC-rCBV-LC images were statistically significant (p-value < 0.001) (e.g. Figure 3), with mean of β 0.59 (range: 0.32 - 1.02).Linear regression analysis of the ASL and DSC perfusion values of all patients showed a very strong linear relationship (R²mean = 0.91 and R²P90 = 0.90, and βmean = 0.60 and βP90 = 0.64) (see Figure 4). Additionally, the correlation analysis of ASL-rCBF and DSC-rCBV-LC showed a very strong correlation for viable tumor (ρmean = 0.88 and ρP90 = 0.91), and perilesional white matter hyperintensity (ρP10 = 0.88) perfusion values.
Discussion
Our study shows a moderate to high positive linear voxel-wise correlation between ASL-rCBF and DSC-rCBV-LC, similar to the work by White et al.2. The two lesions with lowest R² value were both hemorrhagic lesions, which might cause susceptibility artifacts reducing the sensitivity of perfusion by DSC3. Hemorrhage can also cause magnetic field inhomogeneities resulting in image distortion on the EPI sequence4, reducing efficacy of coregistration.For both ASL and DSC measured perfusion values were lowest in the two low grade gliomas, which is in agreement with Warmuth et al.5. However, a clear difference was shown between ASL-rCBF and DSC-rCBV-LC for patients (n = 2) with a medulloblastoma. This might be partially explained by a reduced arterial transit time of lesions in the fossa posterior, resulting in a higher ASL-rCBF value6.
Interestingly, the linear regression coefficients βmean and βP90 approximate the mean value of β (voxel-wise) per patient. This explains why histogram analysis of these lesions might be a good substitute for total tumor perfusion7.
Limitations of this study include a small, heterogeneous patient cohort with a limited number of low-grade tumors and procedural limitations (such as fixed post label delay, no correction of magnetic field distortions, possible registration errors, semi-automatic tumor segmentation and no masking of hemorrhage or macroscopic vessels).
Conclusion
Cerebral blood flow measured with 2D EPI pCASL and leakage corrected, cerebral blood volume measured with DSC show a strong voxel-wise correlation. Therefore, pCASL is a viable alternative to DSC in the workup of new intra-axial brain tumors, especially in patients with contra-indications to intravenous contrast administration.Acknowledgements
No acknowledgement found.References
1. Mutsaerts HJMM, Petr J, Thomas DL, De Vita E, Cash DM, van Osch MJP, et al. Comparison of arterial spin labeling registration strategies in the multi-center GENetic frontotemporal dementia initiative (GENFI). J Magn Reson Imaging. 2018 Jan;47(1):131–40.
2. White CM, Pope WB, Zaw T, Qiao J, Naeini KM, Lai A, et al. Regional and Voxel-Wise Comparisons of Blood Flow Measurements Between Dynamic Susceptibility Contrast Magnetic Resonance Imaging (DSC-MRI) and Arterial Spin Labeling (ASL) in Brain Tumors. J Neuroimaging. 2014;24(1):23–30.
3. Maral H, Ertekin E, Tunçyürek, Özsunar Y. Effects of Susceptibility Artifacts on Perfusion MRI in Patients with Primary Brain Tumor: A Comparison of Arterial Spin-Labeling versus DSC. Am J Neuroradiol. 2020 Feb 1;41(2):255–61.
4. Vardal J, Salo RA, Larsson C, Dale AM, Holland D, Groote IR, et al. Correction of B0-Distortions in Echo-Planar-Imaging–Based Perfusion-Weighted MRI. J Magn Reson Imaging. 2014 Mar 1;39(3):722–8.
5. Warmuth C, Günther M, Zimmer C. Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology. 2003 Aug 1;228(2):523–32.
6. Li X, Sarkar SN, Purdy DE, Briggs RW. Quantifying Cerebellum Grey Matter and White Matter Perfusion Using Pulsed Arterial Spin Labeling. Biomed Res Int. 2014;2014.
7. Arisawa A, Watanabe Y, Tanaka H, Takahashi H, Matsuo C, Fujiwara T, et al. Comparative study of pulsed-continuous arterial spin labeling and dynamic susceptibility contrast imaging by histogram analysis in evaluation of glial tumors. Neuroradiol 2018 606. 2018 Apr 29;60(6):599–608.
Figures