3346
Unmethylated MGMT combined with post-CRT perfusion MRI-derived tumor burden informs overall survival in newly diagnosed glioblastoma1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
As treatment response can mimic progressive tumor on standard imaging, clinical outcomes following upfront chemo-radiotherapy in newly diagnosed glioblastoma rely heavily on MGMT promoter methylation status. While MGMT is a reliable marker of outcomes, the purpose of this study was to determine if inclusion of advanced perfusion-MRI derived fractional tumor burden (FTB) volume might better inform survival outcomes. Results revealed that low FTB volume in MGMT unmethylated glioblastoma confers significant survival benefit, approaching that of MGMT methylated glioblastoma. Clinically, combining FTB with MGMT methylation status may identify patients with potentially better outcomes or for whom more aggressive approaches are warranted.
Introduction
Standard of care for newly diagnosed glioblastoma includes maximum safe resection followed by irradiation with concomitant (CRT) and adjuvant chemotherapy with or without tumor-treating fields1,2. While clinical decision-making relies on a combination of imaging findings and clinical performance, methylation of the MGMT DNA repair enzyme yields more favorable outcomes and greater response to treatment3,4. This difference in response is so great that clinical trials may exclude patients base on MGMT methylation status alone3,4. Beyond MGMT methylation status, early imaging interpretation is also confounded by an inflammatory treatment response which mimics tumor progression as increased contrast enhancement on standard imaging5,6. Advanced perfusion imaging has been recently used to spatially distinguish regions of tumor burden from treatment effect with high accuracy5,6. We therefore hypothesize that early post-CRT DSC-MRI derived fractional tumor burden will inform clinical outcome beyond MGMT methylation status alone.Methods
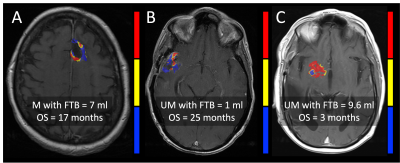
All participants provided written, informed consent according to IRB policy in this retrospective and HIPAA-compliant study. Only those with a newly diagnosed glioblastoma brain tumor following standard upfront therapy including surgical diagnosis followed by CRT and adjuvant temozolomide were considered. Inclusion was limited to those with measurable T1w-enhancing disease, known MGMT promoter methylation status, and pre- and post-contrast T1w and gradient-echo echo-planar dynamic susceptibility contrast (GRE-EPI DSC) MRI acquired within 6 weeks following completion of CRT. DSC MRI was acquired with TE/TR=30/1500 ms, FA=60°, 0.1 mmol/kg preload and 0.1 mmol/kg bolus7. Subjects with known IDH1 mutation were not considered for analysis. FTB maps (using thresholds previously established from MRI spatially co-localized to tumor histology) were generated using leakage corrected and standardized relative cerebral blood volume (rCBV) and standardized deltaT1 maps (Imaging Biometrics, Elm Grove, WI)5,7,8. The volume of perfusion MRI-derived tumor burden was recorded based only on voxels identified as tumor. Twenty-four month overall survival (OS) was evaluated for FTB volume above or below 5 ml (empirically chosen) in combination with MGMT status. Overall survival was estimated using Kaplan-Meier and measured from the date of post-CRT imaging (P<.05 is significant).Results
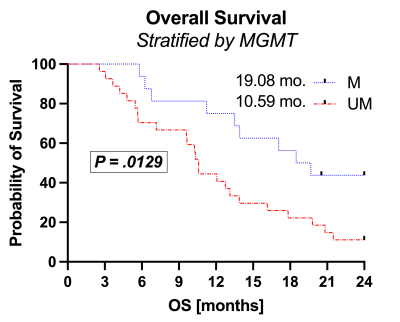
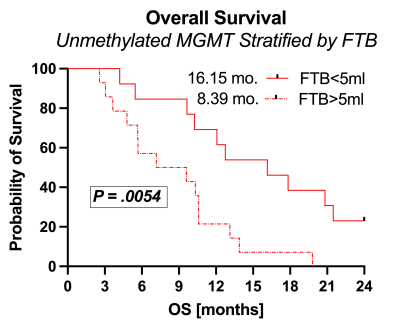
Example FTB maps are shown in Figure 1. Results are shown in Figures 2 and 3. A total of 43 subjects (male/female=21/22) met inclusion criteria, with n=15 MGMT methylated and n=28 MGMT unmethylated. All but one subject was older than 40 y/o (mean=57, range=23-74). Imaging was acquired an average of 29 (range=12-42) days following CRT completion. MGMT methylation status revealed significant OS advantages for methylated (OS=19.08 months) compared to unmethylated (OS=10.18 months) glioblastoma (P=.0129, logrank HR=2.5). While stratification of MGMT methylated glioblastoma by FTB volume (P=0.64) was not significant, MGMT unmethylated glioblastoma with FTB volume less than 5 ml revealed further survival benefit (OS=16.15 mo) over those with FTB greater than 5 ml (OS=8.39 mo) (P=.0054, logrank HR=2.8).Discussion
These results demonstrate that FTB volume in combination with MGMT promoter methylation provides valuable information in the assessment of treatment response within 6-weeks of CRT completion. Low FTB volume may help to further identify MGMT unmethylated subjects with survival advantages (OS=16.15 mo) approaching those of MGMT methylated subjects (19.08 mo). Moreover, those MGMT unmethylated glioblastoma with high FTB volume might benefit from more aggressive treatment strategies or clinical trial placement than those with low FTB volume.Conclusion
Further stratification of MGMT unmethylated glioblastoma by perfusion-derived tumor burden better informs survival outcomes in newly diagnosed glioblastoma patients in the early post-CRT time-period, and may potentially identify those who might respond better to treatment or for whom more aggressive approaches are warranted.Acknowledgements
Funding support provided by NIH/NCI U01 CA176110, NIH/NCI R01 CA255123. FCOI: Imaging Biometrics LLC (KMS-financial interest), IQ-AI Ltd (KMS-ownership interest), Prism Clinical Imaging Inc (KMS-ownership interest).References
1. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. March 2005; 352(10): 987–96.
2. Stupp R, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–16.
3. Li H, Li J, Cheng G, et al. IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg 2016;151:31-36.
4. Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, and Hegi ME. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nature Reviews Neurology volume 6, pages 39–51 (2010)
5. Prah MA, Al-Gizawiy MM, Mueller WM, et al. Spatial discrimination of glioblastoma and treatment effect with histologically-validated perfusion and diffusion magnetic resonance imaging metrics. J Neurooncol. 2018 Jan;136(1):13-21.
6. Hu LS, Eschbacher JM, Heiserman JE, Dueck AC, Shapiro WR, Liu S, Karis JP, Smith KA, Coons SW, Nakaji P, Spetzler RF, Feuerstein BG, Devvins J, and Baxter LC. Reevaluating the imaging definition of tumor progression: perfusion MRI quantifies recurrent glioblastoma tumor fraction, pseudoprogression, and radiation necrosis to predict survival. Neuro Oncol. 2012 Jul;14(7):919-30.
7. Paulson ES, Schmainda KM. Comparison of Dynamic Susceptibility-weighted Contrast enhanced MR Methods: Recommendations for Measuring Relative Cerebral Blood Volume in Brain Tumors. Radiology. 2008;249:601–13.
8. Schmainda KM, Prah MA, Zhang Z, Snyder BS, Rand SD, Jensen TR, Barboriak DP, and Boxerman JL. Quantitative Delta T1 (dT1) as a Replacement for Adjudicated Central Reader Analysis of Contrast-Enhancing Tumor Burden: A Subanalysis of the American College of Radiology Imaging Network 6677/Radiation Therapy Oncology Group 0625 Multicenter Brain Tumor Trial. Am J Neuroradiol. 2019 Jul;40(7):1132-1139.
Figures