3344
Abnormal glutamate metabolism in prefrontal cortex of post-traumatic stress disorder linked to comorbidity with major depression1Biomedical Engineering, Columbia University School of Engineering and Applied Science, New York, NY, United States, 2Radiology and Biomedical Imaging, Yale University School of Medicine, New Haven, CT, United States, 3Clinical Neuroscience Division, Department of Veterans Affairs National Center for Posttraumatic Stress Disorder, Veterans Affairs Connecticut Healthcare System, West Haven, CT, United States, 4Psychiatry, Yale University School of Medicine, New Haven, CT, United States, 5Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, United States, 6Radiology, Columbia University Medical Center, New York, NY, United States, 7Neurology, Yale University School of Medicine, New Haven, CT, United States
Synopsis
Post-traumatic stress disorder (PTSD) is an anxiety condition evidenced by wide-ranging emotional and cognitive dysfunction. While prefrontal glutamatergic excitotoxicity is thought to contribute to its presentation, no 1H-MRS studies of PTSD have yet been conducted at 7-Tesla field strength facilitating separation of glutamate from metabolic partner glutamine. Here we apply 7-T 1H MRS to investigate medial prefrontal (mPFC) concentrations of glutamate and glutamine with GABA, glutathione, and other metabolites in both PTSD and comorbid major depressive disorder (MDD). We show several PTSD-associated abnormalities in mPFC glutamate metabolism that appear to be driven by MDD status when this comorbidity is considered.
Introduction
Post-traumatic stress disorder (PTSD) is an anxiety condition precipitated by stress exposure and manifest as broad dysfunctions in cognition, mood, and arousal1. With an estimated 1 in 12 incidence in some communities2, PTSD may develop in 0.1-19% of trauma-exposed individuals, depending on stressor type3.PTSD symptoms may involve exaggerated glucocorticoid auto-inhibition in the hypothalamic-pituitary-adrenal (HPA) axis, precipitating constitutively low systemic cortisol4. Glutamatergic neurotransmission in fear-conditioning circuitry across the ventromedial and dorsolateral prefrontal cortex, amygdala, and hippocampus5 may also be disrupted by HPA and possibly glial dysfunction, increasing extrasynaptic-to-intrasynaptic glutamate ratios with attendant neuronal excitotoxicity6,7 and observed losses of prefrontal grey-matter volume,8 white-matter integrity,5,9 and fMRI or PET-visible stimulus response1,10.
While proton magnetic resonance spectroscopy (1H-MRS) can measure in vivo glutamate and related metabolites, human 1H-MRS investigations of PTSD are sparse and lack 7-Tesla conditions for separating glutamate from metabolic partner glutamine, unreliable at fields below 4 Tesla11.
We therefore applied 7-T 1H-MRS to measure medial prefrontal cortex (mPFC) metabolites including glutamate, glutamine, neurotransmitter GABA, antioxidant glutathione, and others in individuals with and without PTSD. Because major depressive disorder (MDD) is comorbid with PTSD in an estimated 50% of cases12 and is itself associated with 1H-MRS-visible changes in prefrontal glutamate metabolism13,14, our analysis also included both PTSD and control individuals with MDD.
Methods
We examined N=11 individuals with PTSD (PTSDMDD+ 1F/5M; mean 39 ± S.D. 8 y.o.; PTSDMDD- 2F/3M; 37±16 y.o.) and N=27 trauma-unexposed healthy controls (HCMDD+ 2F/7M; 35±15 y.o.; HCMDD- 5F/13M; 34±8 y.o.) with and without MDD in a one-hour 7-Tesla Yale University Magnetic Resonance Research Center MR scan (Varian Medical Systems, Palo Alto, CA, USA) using eight-channel radiofrequency transception. All participants provided a priori informed consent according to preapproved IRB protocols. Reproducibility-validated acquisition15 included T1-weighted imaging to localize a cubic voxel at the longitudinal fissure of the medial prefrontal cortex (Fig. 1A), local spherical harmonics B0 shimming, global B1 phase shimming, macromolecule-suppressed STEAM (8-cc voxel; TE 10 ms, TM 50 ms, TR 3000 ms, NR 32 x 4-5) for glutamate, and MEGA J-difference-edited semi-LASER (27-cc voxel; TE 72 ms, TR 3000 ms, NR 60 x 2-6) for either GABA or GSH (Fig. 1B).Spectra were anonymized in MATLAB (MathWorks, Natick, MA, USA) to enable blinded INSPECTOR16,17 processing and LCModel18 quantification per reported methods19 (Fig. 2A). Concentrations were referenced to 10 mM total creatine (tCr) and statistics calculated in R (v. 4.0.5)20 as means ± S.D; α = 0.05. One-way ANOVA for PTSD and two-way ANOVA for MDD, PTSD, and MDD x PTSD assessed PTSD effect with and without MDD, respectively. Shapiro-Wilk p>0.05 confirmed ANOVA residual normality before resort to nonparametric analysis. Spearman correlations assessed metabolite relationships to PTSD or MDD severity indices in those with either diagnosis. Supervised classification of disease via support vector machine (SVM) using only prefrontal metabolite concentrations as feature inputs was performed in Python (v. 3.6.13)21 (Fig. 2B).
Results
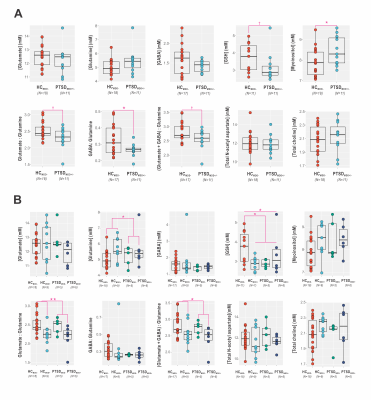
Quantifiable STEAM spectra were acquired for all cases; GABA-JDE, N=37/38, and GSH-JDE, N=29/38 (3.03-ppm creatine full-width at half-maximum FWHM 10.7±1.3 Hz, 12.6±2.4 Hz, and 12.9±2.5 Hz). Clinician-administered PTSD Score (CAPS) did not differ in PTSDMDD+ versus PTSDMDD- groups or correlate with Montgomery-Åsberg Depression Rating Scale (MADRS) in PTSDMDD+ (p>0.1).mPFC glutamate : glutamine (F1,27=3.95, p=0.06) and (glutamate + GABA) : glutamine (F1,27=3.914, p=0.06) trended decreases in PTSD w.r.t. HCMDD- (Fig. 3A) that manifest as MDD effects on glutamine (F1,34=5.723, p=0.02; mean difference M.D. 0.56±0.24 mM, p=0.02), glutamate : glutamine (F1,34=9.801, p=0.004; M.D. -0.27±0.09 mM, p=0.004) and (glutamate + GABA) : glutamine (F1,34=5.737, p=0.02; M.D. -0.26±0.11 mM, p=0.02) once comorbid MDD was considered (Fig. 3B). Additionally, glutathione demonstrated an MDD x PSTD interaction (F1,25=5.477, p=0.03) with decrease over all disease groups w.r.t. HCMDD- (Wilcoxon W=146, p=0.04).
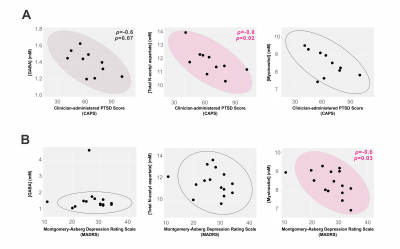
Total N-acetyl aspartate (tNAA) correlated negatively, and GABA showed a trend to negative correlation, with CAPS; myoinositol, with MADRS (Fig. 4).
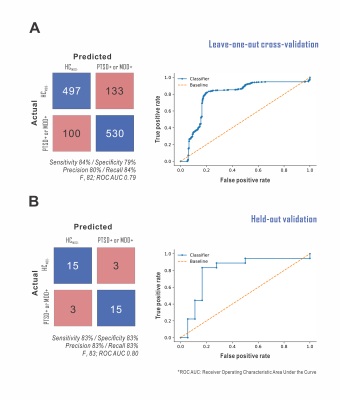
mPFC metabolites alone supported supervised classification of non-HCMDD- disease status with >80% sensitivity and specificity (Fig. 5). Glutamine exhibited the highest permutation importance for this classification.
Conclusions
Here we demonstrate:- PTSD-associated mPFC glutamatergic changes appear associated with comorbid MDD. Glutamine is synthesized from perisynaptic glutamate by nearby glia22. Increased mPFC glutamine, especially relative to glutamate alone or with GABA, may therefore suggest alterations in glutamatergic neurotransmission, with attendant changes in local glial support and associated GABAergic networks. The MDD effect on glutamine concentrations and ratios w.r.t. glutamate with or without GABA exceeded that of PTSD, the latter marginally significant at best and obviated by considering MDD comorbidity.
- mPFC myoinositol concentration, associated with but not fully explained by compensatory gliosis23, correlated negatively with MDD severity.
- Total N-acetyl aspartate concentration in mPFC correlated negatively with PTSD severity, replicating at 7-Tesla field strength multiple reported associations between PTSD symptoms and prefrontal cortex concentrations of this metabolite24,25.
- mPFC metabolite concentrations alone, most importantly glutamine, supported identification of either MDD or PTSD w.r.t. healthy control beyond the 80% sensitivity/specificity threshold previously proposed for diagnostic biomarkers of at least one brain disease26.
Acknowledgements
This research was supported by the VA National Center for PTSD Clinical Neurosciences Division, by the National Institute of Mental Health via award R01MH112668, and by the Yale Center for Clinical Investigation and falls within the purview of Yale Medical School Human Investigation Committee protocols 1501015145, 1305011972, 1402013497 and 1308012549 as well as Columbia University Institutional Review Board protocol AAAR7598. This work was performed in part at the Zuckerman Mind Brain Behavior Institute MRI Platform, a shared resource and Columbia MR Research Center site.References
1. Yehuda, R., Hoge, C. W., McFarlane, A. C., Vermetten, E., Lanius, R. A., Nievergelt, C. M., Hobfoll, S. E., Koenen, K. C., Neylan, T. C. & Hyman, S. E. Post-traumatic stress disorder. Nat Rev Dis Primers 1, 15057, doi:10.1038/nrdp.2015.57 (2015).
2. Bisson, J. I., Cosgrove, S., Lewis, C. & Robert, N. P. Post-traumatic stress disorder. BMJ 351, h6161, doi:10.1136/bmj.h6161 (2015).
3. Kessler, R. C., Aguilar-Gaxiola, S., Alonso, J., Benjet, C., Bromet, E. J., Cardoso, G., Degenhardt, L., de Girolamo, G., Dinolova, R. V., Ferry, F., Florescu, S., Gureje, O., Haro, J. M., Huang, Y., Karam, E. G., Kawakami, N., Lee, S., Lepine, J. P., Levinson, D., Navarro-Mateu, F., Pennell, B. E., Piazza, M., Posada-Villa, J., Scott, K. M., Stein, D. J., Ten Have, M., Torres, Y., Viana, M. C., Petukhova, M. V., Sampson, N. A., Zaslavsky, A. M. & Koenen, K. C. Trauma and PTSD in the WHO World Mental Health Surveys. Eur J Psychotraumatol 8, 1353383, doi:10.1080/20008198.2017.1353383 (2017).
4. Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shin, L. M., Orr, S. P., Gilbertson, M. W., Milad, M. R. & Liberzon, I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13, 769-787, doi:10.1038/nrn3339 (2012).
5. Harnett, N. G., Goodman, A. M. & Knight, D. C. PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Exp Neurol 330, 113331, doi:10.1016/j.expneurol.2020.113331 (2020).
6. Averill, L. A., Purohit, P., Averill, C. L., Boesl, M. A., Krystal, J. H. & Abdallah, C. G. Glutamate dysregulation and glutamatergic therapeutics for PTSD: Evidence from human studies. Neurosci Lett 649, 147-155, doi:10.1016/j.neulet.2016.11.064 (2017).
7. Abdallah, C. G., Averill, L. A., Akiki, T. J., Raza, M., Averill, C. L., Gomaa, H., Adikey, A. & Krystal, J. H. The neurobiology and pharmacotherapy of posttraumatic stress disorder. Annu Rev Pharmacol Toxicol 59, 171-189, doi:10.1146/annurev-pharmtox-010818-021701 (2019).
8. Meng, L., Jiang, J., Jin, C., Liu, J., Zhao, Y., Wang, W., Li, K. & Gong, Q. Trauma-specific grey matter alterations in PTSD. Sci Rep 6, 33748, doi:10.1038/srep33748 (2016).
9. Daniels, J. K., Lamke, J. P., Gaebler, M., Walter, H. & Scheel, M. White matter integrity and its relationship to PTSD and childhood trauma--a systematic review and meta-analysis. Depress Anxiety 30, 207-216, doi:10.1002/da.22044 (2013).
10. Etkin, A. & Wager, T. D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164, 1476-1488, doi:10.1176/appi.ajp.2007.07030504 (2007).
11. Tkáč, I., Oz, G., Adriany, G., Ugurbil, K. & Gruetter, R. In Vivo H-1 NMR spectroscopy of the human brain at high magnetic fields: Metabolite quantification at 4T vs. 7T. Magnet Reson Med 62, 868-879, doi:10.1002/mrm.22086 (2009).
12. Rytwinski, N. K., Scur, M. D., Feeny, N. C. & Youngstrom, E. A. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J Trauma Stress 26, 299-309, doi:10.1002/jts.21814 (2013).
13. Shirayama, Y., Takahashi, M., Osone, F., Hara, A. & Okubo, T. Myo-inositol, glutamate, and glutamine in the prefrontal cortex, hippocampus, and amygdala in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging 2, 196-204, doi:10.1016/j.bpsc.2016.11.006 (2017).
14. Hasler, G., van der Veen, J. W., Tumonis, T., Meyers, N., Shen, J. & Drevets, W. C. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64, 193-200, doi:10.1001/archpsyc.64.2.193 (2007).
15. Prinsen, H., de Graaf, R. A., Mason, G. F., Pelletier, D. & Juchem, C. Reproducibility measurement of glutathione, GABA, and glutamate: Towards in vivo neurochemical profiling of multiple sclerosis with MR spectroscopy at 7T. JMRI 45, 187-198, doi:10.1002/jmri.25356 (2017).
16. Gajdošík, M., Landheer, K., Swanberg, K. M. & Juchem, C. INSPECTOR: free software for magnetic resonance spectroscopy data inspection, processing, simulation and analysis. Sci Rep 11, 2094, doi:10.1038/s41598-021-81193-9 (2021).
17. Juchem, C. INSPECTOR - Magnetic Resonance Spectroscopy Software, <http://innovation.columbia.edu/technologies/cu17130_inspector> (2016).
18. Provencher, S. W. Estimation of metabolite concentrations from localized in-vivo proton NMR-spectra. Magnet Reson Med 30, 672-679, doi:DOI 10.1002/mrm.1910300604 (1993).
19. Swanberg, K. M., Prinsen, H., DeStefano, K., Bailey, M., Kurada, A. V., Pitt, D., Fulbright, R. K. & Juchem, C. In vivo evidence of differential frontal cortex metabolic abnormalities in progressive and relapsing-remitting multiple sclerosis. NMR Biomed 34, e4590, doi:10.1002/nbm.4590 (2021).
20. R Core Team. R: A language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. (2020).
21. Kurada, A. V., Swanberg, K. M., Prinsen, H. & Juchem., C. Diagnosis of multiple sclerosis subtype through machine learning analysis of frontal cortex metabolite profiles. Proc Int Soc Magn Reson Med 4871 (2019).
22. Broer, S. & Brookes, N. Transfer of glutamine between astrocytes and neurons. J Neurochem 77, 705-719, doi:10.1046/j.1471-4159.2001.00322.x (2001).
23. Kim, J. P., Lentz, M. R., Westmoreland, S. V., Greco, J. B., Ratai, E. M., Halpern, E., Lackner, A. A., Masliah, E. & Gonzalez, R. G. Relationships between astrogliosis and 1H MR spectroscopic measures of brain choline/creatine and myo-inositol/creatine in a primate model. AJNR 26, 752-759 (2005).
24. Karl, A. & Werner, A. The use of proton magnetic resonance spectroscopy in PTSD research--meta-analyses of findings and methodological review. Neurosci Biobehav Rev 34, 7-22, doi:10.1016/j.neubiorev.2009.06.008 (2010).
25. Quadrelli, S., Mountford, C. & Ramadan, S. Systematic review of in-vivo neuro magnetic resonance spectroscopy for the assessment of posttraumatic stress disorder. Psychiatry Res Neuroimaging 282, 110-125, doi:10.1016/j.pscychresns.2018.07.001 (2018).
26. Consensus report of the Working Group on: "Molecular and Biochemical Markers of Alzheimer's Disease." The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Neurobiol Aging 19, 109-116 (1998).
Figures