3336
Differential D1 and D2 receptor internalization and recycling induced by amphetamine in vivo: a PET/fMRI study1A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 2Neurobiology Research Unit and Center for Integrated Molecular Brain Imaging, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark, 3Harvard Medical School, Boston, MA, United States
Synopsis
In this study, we imaged the effect of repeated amphetamine injections over >24h using simultaneous PET/fMRI in non-human primates and demonstrate that both internalization and recycling of receptors take place during this timeframe. We provide novel insight into D1 vs. D2 receptor contributions by using a D1 blocking agent in combination with amphetamine injections. These results shed a new light into the timelines of dopamine receptor subtype adaptations and not only provide a link to existing in vitro results but also a foundation for linking receptor-specific mechanisms to initial stages of psychostimulant drug use and abuse.
Introduction
Exposure to psychostimulant drugs like amphetamine can cause short and long-term changes to the dopamine (DA) system. Amphetamine is known to powerfully increase extracellular DA and can rapidly induce receptor internalization1,2. This initial adaptation mechanism in response to drug-induced DA release that can have lasting effects of several hours up to days. Reductions in dopamine D2/D3 receptor (D2/D3R) availability, measured using positron emission tomography (PET), has been a robust finding in stimulant drug users. Yet, microdialysis and functional imaging studies have also reported discrepancies between the expected timeline of DA release/uptake and paradoxically prolonged reductions in PET signal3,4. In vitro data have suggested that the rate of receptor recycling can vary between the D1 and the D2R subtypes5, yet, little is known about the functional effects in vivo. In this study, we investigated the functional consequences of differential D1 and D2R trafficking mechanisms following stimulant exposure by imaging with combined PET/fMRI during repeated amphetamine injections in non-human primates.Methods
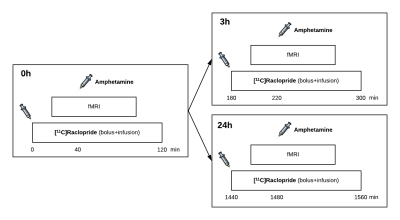
Simultaneous PET/fMRI was acquired in three anesthetized rhesus macaques in a total of 20 imaging sessions: The D2/D3R PET radiotracer [11C]raclopride was administered as a bolus+infusion, while gradient-echo EPI was acquired continuously with a total scan duration of 2h. Ferumoxytol was injected before the start of fMRI acquisitions as a contrast agent. In 14 sessions, a first (0h) amphetamine dose (0.6 mg/kg) was given intravenously at 40 min after radiotracer administration as a within-scan challenge, followed by a second amphetamine injection after 3h or 24h using the same PET/fMRI acquisitions (Fig. 1). In an analogous set of 6 imaging sessions, SCH23390 was administered as a bolus+infusion (0.1 mg/kg + 0.09 mg/kg/h) to block D1R prior to radiotracer injection. FMRI data were analyzed with a general linear model and converted to relative changes in cerebral blood volume (CBV). Binding potential (BPND) and receptor occupancy were quantified using a modified simplified reference tissue model with a time-dependent binding term6,7.Results
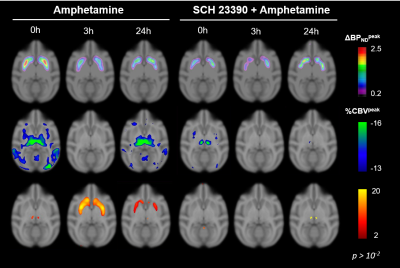
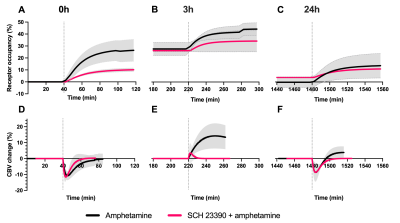
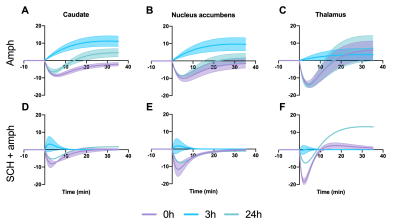
After each amphetamine injection, a reduction in striatal [11C]raclopride-PET binding was observed, driven by amphetamine-induced DA release (Fig. 2). Amphetamine-induced CBV changes demonstrated both a positive and negative CBV component that were modulated and interestingly shifted in sign with repeated injections, as seen on the voxelwise maps (Fig. 2). For the first 0h amphetamine injection, we observed a reduction in [11C]raclopride BPND, equivalent to a peak occupancy of 27.3% [19.1; 35.1] (mean, [95% confidence interval]), together with a short-lived decrease in CBV with a negative peak at -11.2% [-13.4; -9.0] in the putamen (Fig. 3A,D, black lines). The repeated amphetamine administration after 3h caused a further reduction in BPND, i.e. an increase in D2/D3R occupancy with combined peak occupancy of 46.6% [37.2; 94.6] (Fig. 3B). In contrast to the first drug injection, the CBV response at 3h showed an entirely different functional response: a long-lasting increase in CBV with a peak change of 14.2% [-5.4; 33.9] (Fig. 3E, black lines). At 24h, D2/D3R occupancy had returned to baseline, but showed reduced acute amphetamine-induced occupancy of 13.9% [2.2; 25.6]. The repeated amphetamine administration at 24h caused a biphasic CBV response, consisting of a negative component (-7.3% [-14.8; 0.2]), similar to the 0h response, but exhibiting a longer-lasting positive component (4.1% [-1.3; 9.6]), as seen with the amphetamine injection at 3h (Fig. 3C,F, Fig. 4A-C).Administration of SCH23390 neither altered the overall shape of the amphetamine-induced changes in CBV at 0h (Fig. 3D, pink lines), nor appreciably affected BPND. Most interestingly, the long-lasting positive CBV response at 3h was reduced to a short-lived positive response (Fig. 3E, pink lines), demonstrating the D1-specificity of the functional response. At 24h, the positive, excitatory component of the CBV response was also attenuated, further providing evidence of the D1 component of the response. Overall, CBV responses behaved similarly in regions of the basal ganglia (putamen, caudate, nucleus accumbens). The thalamus exhibited a slightly different CBV response, with a biphasic CBV response at both 0h and 24h, and a more moderate positive CBV signal at repeated amphetamine injections that was not eliminated by SCH23390 blocking at 24h (Fig. 4).
Discussion
The results from this combined PET/fMRI study demonstrate (i) persistent D2/D3 receptor internalization after even just one amphetamine exposure at 0h that lasts for up to 24h, (ii) continued DA release with repeated injections and (iii) differences in functional activation between D1 and D2/D3R within 3h of repeated amphetamine exposure. The elimination of the dominant positive CBV response at 3h after D1 receptor blocking demonstrates the D1-specificity of the functional response. Our data suggest that excitatory vs. inhibitory DA receptor subtypes have varying time constants for internalization and recycling, with inhibitory D2/D3R taking a longer time to recycle/externalize compared to D1 receptors after amphetamine exposure. We present these novel results on the modulation of both D1 vs. D2R activity due to repeated amphetamine in a translational in vivo setting. Our data uniquely show the adaptation of receptors over time in vivo, thereby providing insight into adaptive changes beyond the D2/D3 receptors, and the potential role of D1R that may be of relevance for understanding the molecular mechanisms of stimulant use and abuse.Acknowledgements
This work was supported by NIH grants K99DA043629, R00DA043629, P41EB015896, S10RR026666, S10RR022976, S10RR019933 and S10RR017208.References
1. Narendran R, Slifstein M, Hwang D-R, et al. Amphetamine-induced dopamine release: Duration of action as assessed with the D2/3 receptor agonist radiotracer (––)-N-[11C]propyl-norapomorphine ([11C]NPA) in an anesthetized nonhuman primate. Synapse 2007; 61: 106–109.
2. Skinbjerg M, Liow J-S, Seneca N, et al. D2 dopamine receptor internalization prolongs the decrease of radioligand binding after amphetamine: A PET study in a receptor internalization-deficient mouse model. Neuroimage 2010; 50: 1402–1407.
3. Laruelle M, Iyer RN, Al-Tikriti MS, et al. Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 1997; 25: 1–14.
4. Liu CH, Greve DN, Dai G, et al. Remifentanil administration reveals biphasic phMRI temporal responses in rat consistent with dynamic receptor regulation. Neuroimage 2007; 34: 1042–1053.
5. Bartlett SE, Enquist J, Hopf FW, et al. Dopamine responsiveness is regulated by targeted sorting of D2 receptors. Proc Natl Acad Sci U S A 2005; 102: 11521–11526.
6. Ichise M, Liow J-S, Lu J-Q, et al. Linearized Reference Tissue Parametric Imaging Methods: Application to [11C]DASB Positron Emission Tomography Studies of the Serotonin Transporter in Human Brain. J Cereb Blood Flow Metab 2003; 23: 1096–1112.
7. Sander CY, Hooker JM, Catana C, et al. Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci U S A 2013; 110: 11169–74.
Figures