3334
Comparing the efficiency of data-driven noise regression in removing cardiac and respiratory signals from rs-fMRI: Difference across age groups1University of Toronto, Toronto, ON, Canada, 2Rotman Research Institute at Baycrest, Toronto, ON, Canada, 3Department of Biophysics, University of Toronto, Toronto, ON, Canada
Synopsis
Data-driven methods have been suggested to remove heartbeat and respiration noises from fMRI signals. We compared the effectiveness of these methods (global-signal regression (GS), white matter and CSF (cerebrospinal fluid) regression, anatomical and temporal CompCor, ICA AROMA) in removing the noise. GS, AROMA, and aCompCor removed the most physiological fluctuation, but GS and AROMA also removed most signals under 0.1 Hz. We also observed that all methods removed less noise power and more low-frequency power from young adult data compared to older adults.
Introduction
Fluctuations in blood flow and oxygenation due to cardiac and respiratory effects is a major source of noise in fMRI data1. Recording physiological signals can be challenging, unreliable, or impossible2, so several data-driven methods have been proposed to remove the physiological noise from data. The efficacy of these noise removal techniques however is not clear. Since the typical sample rate of the fMRI data is above the nyquist frequency of the respiration and heartbeat, these signals alias into low frequencies, and therefore investigating the efficacy of the noise removal techniques is challenging. We used fMRI data with high temporal resolution with whole-brain coverage and simultaneously recorded physiological signals to evaluate the performance of several data-driven noise removals. The high-temporal resolution allows direct visualization of the power spectra of noise peaks pre and post denoising. Given that physiological contributions may vary with age3, we compare the performance of these denoising methods across young and older subjects.Method
18 healthy young subjects (age=26.7 ± 6.5 years) and 18 healthy older subjects (age=74.2 ± 7.0 years) were scanned using a Siemens TIM Trio 3T scanner. rs-fMRI scans were collected using SMS-EPI BOLD (TR/TE = 380/30 ms, FA = 40°, 20 5-mm slices, 64x64 matrix, 4x4x5 mm voxels, MB = 3, 1,950 volumes). Cardiac pulsation was recorded using the scanner pulse oximeter, whereas the respiratory signal was recorded using a BiopacTM system. Subject-specific heartbeat and respiration frequency is estimated based on the peak in the spectrum of the physiological signals. The rs-fMRI preprocessing was implemented through fMRIPrep, and includes motion correction, spatial smoothing (5mm FWHM), high-pass filtering (>0.01 Hz) and brain extraction. This is a common processing pipeline applied prior to all physiological denoising methods.Physiological denoising: Five methods of noise correction are included in the comparison, as implemented through fMRIPrep6: global signal regression (GS), white matter and CSF (cerebrospinal fluid) signal regression (WM-CSF), anatomical CompCor (aCompCor)4, temporal CompCor (tCompCor)4, and ICA AROMA5.
Evaluation metrics: The fMRI signal is averaged across the gray matter and white matter of each individual and the spectrum of the averaged signals is calculated. We computed the total fMRI signal power pre- and post-noise correction in three frequency bands: (1) a 0.1 Hz band centered at the subject-specific heartbeat; (2) a 0.2 Hz band centered around each subject’s respiration frequency; (3) the frequency band between 0.01 and 0.1 Hz, referred to as the “low-frequency band”. To evaluate the extent to which each denoising method alters the contribution of these frequency bands, , we computed the fractional power change as:
(Puncorrected - Pcorrected) / Puncorrected (1)
Where Puncorrected is the power of the fMRI signal before noise correction in one of the three frequency bands and Pcorrected is the power of the fMRI signal after noise correction in these frequency bands. The use of fractional spectral-power change allows direct comparison across age groups, especially given aging-related total power decrease7, A successful noise removal would have high fractional power removed from the cardiac and respiratory frequency and low fractional power removed in the low frequency band.
Results
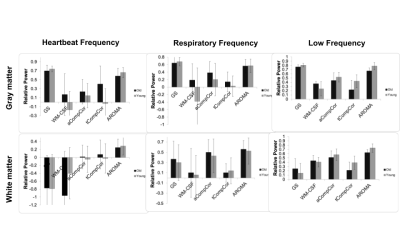
Figure 1 shows the fractional power changes in heartbeat (left), respiration (middle), and low-frequency (right) for the gray matter (top) and while matter (bottom) signals. Young and older adults are shown grey and black, respectively. All methods resulted in spectral-power reduction (as high as 60-70%) in all three frequency bands of the grey matter signal, with GS and AROMA removing the most noise power, followed by aCompCor. GS and AROMA also removed the most signal from the low-frequency band (up to 80%). All methods are less effective against heart-rate related signal power in the white matter, where AROMA also removed the most signal from (>70%). Moreover, all methods removed less noise power from the data of young adults compared to older adults. Conversely, all methods removed more low-frequency power from the data of young adults.Discussion
The amplitude of low-frequency fluctuation decreases with advancing age7, so one can expect the low-frequency band to contribute less to the total rs-fMRI signal power, and the denominator of Eq. (1) to be lower in the older adults, resulting in higher relative noise power change by denoising. Overall, AROMA and GS consistently remove more heart-beat and respiratory frequencies, but also the most low-frequency signals, which may suggest removal of neuronally-relevant signals. aCompCor seems to offer a good compromise, but it performs very differently from WM-CSF regression, contrary to common assumption.Conclusion
The findings of this study directly influence the interpretation of age effects on rs-fMRI metrics, suggesting that age effects in rs-fMRI signal power calculated following different denoising methods cannot be directly compared. In future we will also investigate the effect of the noise removal methods on the resting-state connectivity maps.Acknowledgements
No acknowledgement found.References
1. Liu, T. T. Noise contributions to the fMRI signal: An overview. Neuroimage 143, 141–151 (2016).
2. Agrawal, U., Brown, E. N. & Lewis, L. D. Model-based physiological noise removal in fast fMRI. Neuroimage 205, 116231 (2020).
3. Van Dijk, K. R. A., Sabuncu, M. R. & Buckner, R. L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438 (2012).
4. Behzadi, Y., Restom, K., Liau, J. & Liu, T. T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101 (2007).
5. Pruim, R. H. R. et al. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277 (2015).
6. Esteban, O. et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116 (2019).
7. Hu, S., Chao, H. H.-A., Zhang, S., Ide, J. S. & Li, C.-S. R. Changes in cerebral morphometry and amplitude of low-frequency fluctuations of BOLD signals during healthy aging: correlation with inhibitory control. Brain Struct. Funct. 219, 983–994 (2014).