3330
Improving fMRI acquisition using single-shot EPTI with distortion-free high-SNR high-CNR multi-echo imaging1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Department of Radiology, Stanford University, Stanford, CA, United States, 4Department of Electrical Engineering, Stanford University, Stanford, CA, United States
Synopsis
This work aims to address the limitations of EPI and improve fMRI acquisition by developing a single-shot EPTI method, to provide fast distortion-free imaging, with significantly improved tSNR, CNR, robustness to motion, reduced signal dropout, and multi-echo capability for denoising/analysis. The single-shot acquisition is enabled by the newly-designed k-t encoding to provide stronger correlation for improved reconstruction. Results show ss-EPTI achieves: i) 30% higher tSNR efficiency than EPI and 70% higher than 3-shot EPTI; ii) 50% higher CNR than EPI (visual-task); iii) distortion-free fMRI with improved robustness to motion; iv) effective signal dropout reduction; and v) effective non-BOLD variation separation.
Introduction
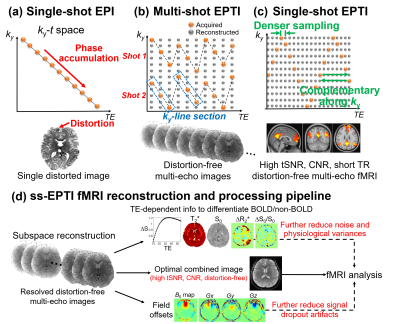
Echo-planar imaging (EPI)1 has been the standard acquisition for fMRI due to its high efficiency. However, its limitations such as distortion artifacts, signal dropout, suboptimal CNR, and vulnerability to physiological noise, introduce bias and undesired variances that complicate fMRI analysis. fMRI using multi-echo EPI acquisition2-4 has been shown to improve BOLD CNR, mitigate signal dropout, and enable effective removal of non-BOLD physiological noise2,3,5.In this work, we will address EPI’s limitation and improve fMRI acquisition based on our recently developed technology, Echo Planar Time-resolved Imaging (EPTI)6, and further develop it into a single-shot scheme (ss-EPTI) to provide distortion-free multi-echo imaging at high temporal resolution, with increased robustness to motion and physiological noise, and higher tSNR and CNR efficiency over EPI acquisition. EPTI exploits spatiotemporal correlation in the encoding process and resolves distortion-free multi-echo images from fully-recovered spatiotemporal space. The single-shot acquisition is enabled by the newly-designed k-t (TE) encoding that reduces echo-spacing by ~40% to provide stronger temporal correlation for improved reconstruction. The continuous ss-EPTI readout starts from 3-4ms with minimal deadtime, enabling high sampling efficiency to increase SNR efficiency. In each TR, it can resolve ~120 echo whole-brain volumes spaced at an echo spacing (~0.5ms), which can be used to achieve optimal CNR and effectively recover signal dropout. By combining with ME-ICA2,3,7,15, the multi-echo information was used to identify non-BOLD variations for motion/physiological noise reduction2,3,7,15. We have also incorporated intra-voxel dephasing correction8 into EPTI. The preliminary results show ss-EPTI provides: i)~30% higher tSNR efficiency than EPI and ~70% higher than conventional 3-shot EPTI; ii) images free from geometric and dynamic distortion, enabling improved temporal stability even with more severe subject motion; iii) improved CNR by 50% over EPI (visual-task), iv) effectively reduced signal dropouts, and v) non-BOLD variation separation from the BOLD activation using ME-ICA2,3,7,15.
Methods
Single-shot EPTI acquisition: Conventional EPI generates a single distorted image after combining k-space samples evolving across time with accumulated B0 phase (Fig.1a). EPTI uses spatiotemporal encodings (Fig.1b) to exploit the data correlation and fully reconstruct the k-t (TE) space, generating >100 distortion-free multi-echo image across EPTI readout. Original EPTI encoding acquires different ky-line-sections to ensure regular sampling to apply GRAPPA kernel6, which requires large gradient blips especially for single-shot acquisition, leading to deadtime and compromised data correlation. To achieve single-shot EPTI acquisition, we employ a zig-zag trajectory (Fig.1c) that continuously traverses through the k-t(TE) space with minimal gradient blips required, therefore reducing the deadtime and achieving much shorter echo-spacing with stronger spatiotemporal correlation for improved reconstruction (~40% denser sampling, echo-spacing 0.93ms->0.55ms at 2-mm). The samplings are complementary along ky to better use the multi-channel coil information9,10. Simultaneous-multi-slice (SMS)11 imaging was also integrated to increase temporal resolution.The ss-EPTI reconstruction and processing pipeline for fMRI is shown in Fig.1d. First, a multi-echo subspace reconstruction12,13 will be employed to exploit the correlation in the k-t (TE) space and resolve multi-echo images completely free from static and dynamic distortions. The multi-echo images will then be combined based on the T2* of every voxel to generate CNR-optimal images14. We further incorporated intra-voxel dephasing correction8 to reduce the dropout artifacts. Additionally, the multi-echo images can be used to extract TE-dependency information to identify and remove non-BOLD variations for motion and physiological-noise/artifacts reduction using ME-ICA2,3,7,15.
Visual-task and resting-state fMRI experiments were performed on healthy volunteers using ss-EPTI and standard ss-EPI (see figure caption for protocols). Note that tSNR&CNR were evaluated without denoising/smoothing.
Results
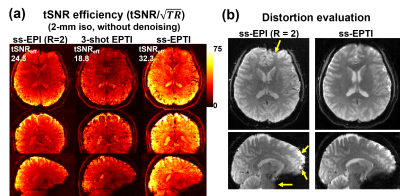
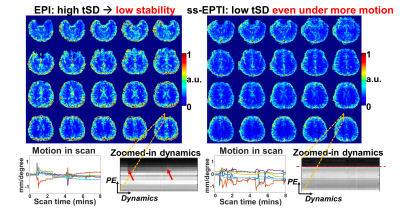
Fig.2a shows the tSNR efficiency (tSNR/√TR) of conventional ss-EPI (TR minimized), 3-shot EPTI, and ss-EPTI. ss-EPTI provides 30% higher tSNR efficiency over ss-EPI and 70% higher than 3-shot EPTI, due to its high sampling efficiency (~2x more sampling time than ss-EPI /TR with only ~20% increase in TR) and increased robustness to motion. Fig2.b shows that ss-EPTI eliminates geometric distortion common in ss-EPI even with short effective echo-spacing.The elimination of motion-related dynamic distortion of ss-EPTI provides higher temporal stability (Fig.3) even with more subject motion. The example zoomed-in area shows the local variation of the brain contour in ss-EPI and static image geometry of ss-EPTI.
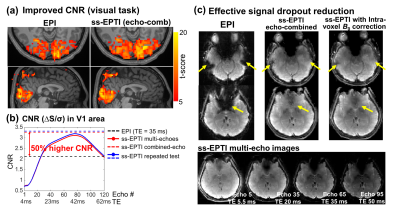
In visual-task experiment, ss-EPTI shows improved CNR than EPI with higher t-score (Fig.4a). The optimal echo-combined EPTI images provide 50% higher CNR in V1 area. The dense EPTI multi-echo images enable investigation of CNR across TEs (Fig.4b), peaked at TE=42ms in V1 area. A repeated EPTI run was tested, and consistent results were found. Fig.4c presents the effectively reduced signal dropout using ss-EPTI, benefiting from the acquired early-TE images and intra-voxel dephasing correction.
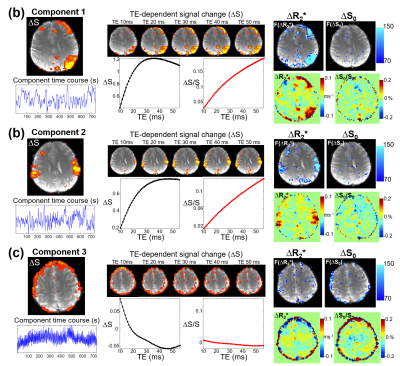
ss-EPTI multi-echo images can be used to investigate TE-dependency and differentiate BOLD/non-BOLD components for noise removal. The signal (ΔS shown in black curve or ΔS/S in red) change differently across echoes depending on the S0 (non-BOLD) and R2* (BOLD) change. For BOLD components (Fig.5a&b, strong R2* change), percent signal change (ΔS/S) changes linearly with TE, while the non-BOLD component (Fig.5c, strong S0 change) is more constant across TEs. The densely-sampled multi-echo images also allow analysis of peak echo-time for different BOLD components.
Conclusion
Single-shot EPTI improves the fMRI acquisition by providing high-quality distortion-free images, with higher tSNR, CNR efficiency, temporal stability, reduced signal dropout, and multi-echo capability for denoising.Acknowledgements
This work was supported by the NIH NIBIB (R01-EB019437, P41EB030006) and the instrumentation Grants (S10-RR023401, S10-RR023043, and S10-RR019307).References
1 Mansfield, P. Multi-Planar Image-Formation Using Nmr Spin Echoes. Journal of Physics C-Solid State Physics 10, L55-L58, doi:Doi 10.1088/0022-3719/10/3/004 (1977).
2 Kundu, P. et al. Multi-echo fMRI: A review of applications in fMRI denoising and analysis of BOLD signals. NeuroImage 154, 59-80, doi:10.1016/j.neuroimage.2017.03.033 (2017).
3 Kundu, P., Inati, S. J., Evans, J. W., Luh, W. M. & Bandettini, P. A. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage 60, 1759-1770, doi:10.1016/j.neuroimage.2011.12.028 (2012).
4 Poser, B. A. & Norris, D. G. Investigating the benefits of multi-echo EPI for fMRI at 7 T. NeuroImage 45, 1162-1172, doi:10.1016/j.neuroimage.2009.01.007 (2009).
5 Evans, J. W., Kundu, P., Horovitz, S. G. & Bandettini, P. A. Separating slow BOLD from non-BOLD baseline drifts using multi-echo fMRI. NeuroImage 105, 189-197, doi:10.1016/j.neuroimage.2014.10.051 (2015).
6 Wang, F. et al. Echo planar time-resolved imaging (EPTI). Magnetic resonance in medicine 81, 3599-3615, doi:10.1002/mrm.27673 (2019).
7 Kundu, P. et al. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences 110, 16187-16192 (2013).
8 Lam, F. & Sutton, B. P. Intravoxel B0 inhomogeneity corrected reconstruction using a low-rank encoding operator. Magnetic resonance in medicine 84, 885-894, doi:10.1002/mrm.28182 (2020).
9 Breuer, F. A. et al. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magnetic resonance in medicine 55, 549-556, doi:10.1002/mrm.20787 (2006).
10 Dong, Z. et al. Tilted-CAIPI for highly accelerated distortion-free EPI with point spread function (PSF) encoding. Magnetic resonance in medicine 81, 377-392, doi:10.1002/mrm.27413 (2019).
11 Setsompop, K. et al. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magnetic resonance in medicine 67, 1210-1224, doi:10.1002/mrm.23097 (2012).
12 Dong, Z., Wang, F., Reese, T. G., Bilgic, B. & Setsompop, K. Echo planar time-resolved imaging with subspace reconstruction and optimized spatiotemporal encoding. Magnetic resonance in medicine 84, 2442-2455, doi:10.1002/mrm.28295 (2020).
13 Liang, Z.-P. in 2007 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro. 988-991 (IEEE).
14 Gowland, P. A. & Bowtell, R. Theoretical optimization of multi-echo fMRI data acquisition. Phys Med Biol 52, 1801-1813, doi:10.1088/0031-9155/52/7/003 (2007).
15. The tedana Community, Ahmed, Zaki, Bandettini, Peter A., Bottenhorn, Katherine L., Caballero-Gaudes, César, Dowdle, Logan T., DuPre, Elizabeth, Gonzalez-Castillo, Javier, Handwerker, Dan, Heunis, Stephan, Kundu, Prantik, Laird, Angela R., Markello, Ross, Markiewicz, Christopher J., Maullin-Sapey, Thomas, Moia, Stefano, Salo, Taylor, Staden, Isla, Teves, Joshua, Uruñuela, Eneko, Vaziri-Pashkam, Maryam, Whitaker, Kirstie. ME-ICA/tedana: 0.0.11. Zenodo; 2021.
Figures