3329
Functional MRI with three-dimensional reduced field-of-view1Center for MR Research, University of Illinois at Chicago, Chicago, IL, United States, 2Department of Biomedical Engineering, University of Illinois at Chicago, Chicago, IL, United States, 3Departments of Radiology and Neurosurgery, University of Illinois at Chicago, Chicago, IL, United States
Synopsis
Functional MRI (fMRI) is typically performed with whole brain coverage, even when only a small brain area is of interest. Although zoomed fMRI has been demonstrated using 2D multi-slice acquisitions, extending this capability to 3D focused volume has not been well explored. We have implemented a 3D reduced field-of-view technique for fMRI by using a 2D RF pulse and applied this technique to a visual fMRI study on ten subjects. Compared to a conventional fMRI sequence with a full field-of-view, our technique produced high isotropic spatial resolution and reduced image distortion, despite a moderate reduction in temporal signal-to-noise ratio.
Introduction:
Function MRI (fMRI) is typically performed with a field-of-view (FOV) covering the entire brain. When only a specific brain area (or areas) is of interest, fMRI can be performed over a reduced FOV to increase the spatial resolution, shorten the TR, and/or reduce image distortion 1–3. Existing techniques, however, are limited to two-dimensional (2D) multi-slice imaging. Recently, a three-dimensional reduced field-of-view imaging (3D-rFOVI) technique was reported, allowing zoomed acquisition in all three directions 4. This capability can enhance 3D fMRI acquisitions, as 3D fMRI is becoming increasingly popular 5–7. The purpose of the present study is to apply 3D rFOVI to a gradient-echo EPI (GRE-EPI) sequence for high-resolution 3D fMRI over a zoomed volume. To demonstrate its advantage, the results from 3D-rFOVI are compared with those from conventional 3D full-FOV GRE-EPI.Methods:
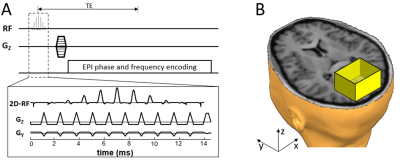
3D-rFOVI sequence:3D-rFOVI employs a 2D RF pulse to excite a slab along the conventional slice-selection direction (i.e., z-direction) while limiting the spatial extent along the phase-encoded direction (i.e., y-direction) within the slab (Figure 1A). The FOV along the readout direction (i.e., x-direction) is restricted by taking advantage of the anti-aliasing filter in the RF receiver system (Figure 1B). A stepping phase-encoding gradient is applied to provide spatial encoding along the z-direction, while in-slab phase-encoding is accomplished by the EPI blip gradient along the y-direction. 3D r-FOVI can produce isotropic high spatial resolution and reduce image distortion caused by off-resonance effects, such as magnetic susceptibility variations. In our design, the 2D RF pulse employed eleven sub-pulses, each with a time-bandwidth product (TBP) of 3.01. The amplitude of these sub-pulses was modulated by an envelope RF pulse whose TBP was 3.53 and pulse width was 14.7 ms (Figure 1A). Both the sub-pulses and the envelope pulse were designed using a Shinnar-Le-Roux algorithm with a linear phase 8. An EPI-like fly-back gradient was played out concurrently with the RF pulses to traverse excitation k-space while avoiding Nyquist ghosts 1,9. The main excitation band in the spatial response of the 2D RF pulse was used for 3D volume localization, and the side bands were positioned outside the imaged object.
Image acquisition and processing:
The 3D-rFOVI technique was implemented in a GRE-EPI sequence on a GE MR750 3T scanner with a 32-channel head coil. In the fMRI experiments with visual stimulation, ten healthy human subjects (4 female, 6 male; 29.4 ± 6.1 years) were scanned using the 3D-rFOV GRE-EPI sequence with the following parameters: TR/TE = 100/30 ms, TRvol = 2 s, number of volumes = 120, flip angle = 20°, FOV = 120×120 mm2, slab thickness = 38 mm, acquisition matrix = 64×64×20, and isotropic spatial resolution = 1.9×1.9×1.9 mm3. For comparison, images in the same imaging slab were also acquired using a 3D GRE-EPI sequence with a full FOV of 240×240×38 mm3 and an acquisition matrix of 128×128×20. Visual stimulation was delivered with a dark-gray and light-gray checkboard pattern flashing at 8 Hz. Our block-design paradigm contained six 40 s blocks, each with a 20 s stimulation followed by a 20 s rest. The total acquisition time was 4 min. Subjects were asked to fixate on the cross-hair presented at the center of visual field during the experiment. All data were analyzed using SPM8 on MATLAB 2021a. Data preprocessing included image realignment and spatial smoothing with a 3 mm full-width-at-half-maximum Gaussian kernel. A general linear model was applied for visual activation detection with a threshold of P < 0.05 (FWE-corrected) and spatial cluster size of at least 30 pixels.
Results:
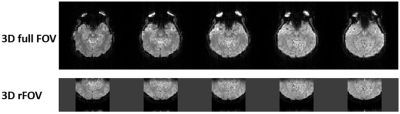
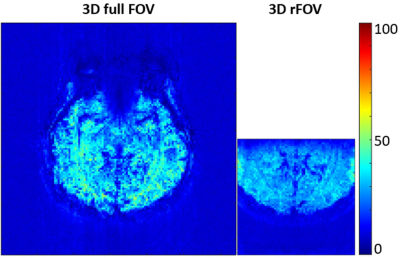
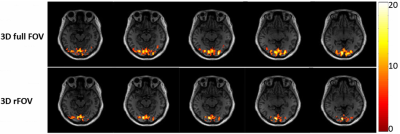
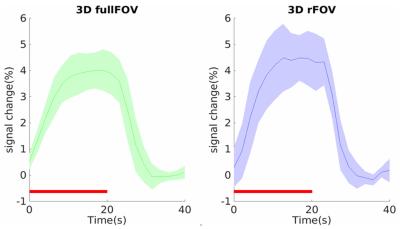
A set of human brain images acquired using 3D full-FOV and 3D-rFOV GRE-EPI are displayed in Figure 2. 3D-rFOVI successfully excited a zoomed volume at the virtual cortex, where virtually no geometric distortion was observed. Compared with 3D full-FOV imaging, 3D-rFOVI reduced temporal SNR (tSNR) in the visual cortex by ~13% (Figure 3). Figure 4 illustrates five contiguous visual fMRI activation maps from a presentative subject for the two sequences. Decreased activated volume was detected in 3D-rFOV GRE-EPI, which was a reflection of reduced tSNR due to fewer k-space sampling points. The average time courses across the ten subjects at the visual cortex are shown in Figure 5, where the 3D-rFOV sequence produced similar BOLD signal changes (~4%) as the 3D full-FOV sequence.Discussion and Conclusion:
We have demonstrated a novel 3D-rFOV fMRI technique that targets at a specific 3D functional region in the brain. Compared with 3D full-FOV fMRI, the new strategy can produce isotropic high spatial resolution while providing resilience to geometric distortion. A downside was the reduced activated volume, likely due to the lower tSNR as a result of reduced k-space sampling. The benefits of using 3D-rFOV for fMRI are expected to be greater at a higher magnetic field where the need for image distortion reduction is more prominent due to the increased off-resonance effects and where the improved tSNR can better support zoomed fMRI activation detection.Acknowledgements
This work was supported in part by the National Institutes of Health (5R01EB026716-01 and 1S10RR028898-01) and a Chancellor’s Translational Research Initiative (CTRI) Grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. Y. Sui of Mayo Clinic for helpful discussions and the artwork used in Figure 1B.References
1. Finsterbusch J. Functional neuroimaging of inner fields-of-view with 2D-selective RF excitations. Magn Reson Imaging. 2013;31(7):1228-1235.
2. Islam H, Glover GH. Reduced field of view imaging using a static second-order gradient for functional MRI applications. Magn Reson Med. 2016;75(2):817-822.
3. Karaman MM, Sui Y, Xiong Y, and Zhou XJ. fMRI in All Plane Orientations with Decreased Image Distortion Using A 2D RF Pulse for Field-of-View Reduction. Proceedings of the 102nd Annual Meeting of the RSNA. 2016; SSA22-08.
4. Sun K, Zhong Z, Dan G, Karaman M, Luo Q, Zhou XJ. Three-dimensional reduced eld-of-view imaging (3D-rFOVI). ISMRM. 2021; p4193
5. Goerke U, Moller HE, Norris DG, Schwarzbauer C. A comparison of signal instability in 2D and 3D EPI resting-state fMRI. NMR Biomed. 2005;18(8):534-542.
6. Afacan O, Hoge S, Brooks DH, Mórocz IÁ. Increasing temporal resolution in hybrid 3D EPI FMRI studies using unfold. IEEE International Symposium on Biomedical Imaging: From Nano to Macro. 2009.
7. Lutti A, Thomas DL, Hutton C, Weiskopf N. High-resolution functional MRI at 3 T: 3D/2D echo-planar imaging with optimized physiological noise correction. Magn Reson Med. 2013;69(6):1657-1664.
8. Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm. IEEE Trans Med Imaging. 1991;10(1):53-65.
9. Zhong Z, Merkitch D, Karaman MM, Zhang JX, Sui Y, Goldman JG, Zhou XJ. High-spatial-resolution diffusion MRI in Parkinson disease: lateral asymmetry of the substantia nigra. Radiology. 2019;291(1):149-157.
Figures