3326
NORDIC PCA increases tSNR in both human and mouse resting-state fMRI for potential improvements in cerebrovascular reactivity mapping
Emily L. Tse1, Russell W. Chan1,2, Sarah Y. Wu1, Yixi Xue1, Peiying Liu3, Steen Moeller4, and Kevin C. Chan1,5
1Department of Ophthalmology, New York University Grossman School of Medicine, New York, NY, United States, 2Neuroscience Institute, New York University Grossman School of Medicine, New York, NY, United States, 3Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 4Center for Magnetic Resonance Research (CMRR), University of Minnesota, Minneapolis, MN, United States, 5Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States
1Department of Ophthalmology, New York University Grossman School of Medicine, New York, NY, United States, 2Neuroscience Institute, New York University Grossman School of Medicine, New York, NY, United States, 3Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 4Center for Magnetic Resonance Research (CMRR), University of Minnesota, Minneapolis, MN, United States, 5Department of Radiology, New York University Grossman School of Medicine, New York, NY, United States
Synopsis
Cerebrovascular reactivity (CVR) reflects the response of cerebral blood vessels to vasoactive stimuli. Whole-brain relative CVR (rCVR) mapping is achieved using task-free resting-state fMRI, which resembles conventional CVR mapping using hypercapnia challenge. To optimize rCVR mapping, we apply NOise Reduction with DIstribution Corrected (NORDIC) PCA which removes thermal noise originating from the scanner and/or the subject. Results show that NORDIC-correction increases the temporal signal-to-noise ratio in both humans and mice, lowers mouse rCVR variance, and potentially improves mouse rCVR along cortical layers. Future rCVR studies investigating cerebrovascular diseases may incorporate NORDIC-correction for higher sensitivity.
Introduction
Cerebrovascular reactivity (CVR) reflects the response of cerebral blood vessels to vasoactive stimuli1, and may be indicative of cerebrovascular health in many conditions and pathologies2, including stroke3 and dementia4. Conventional whole-brain CVR mapping utilizes hypercapnic gas inhalation or breath-holding tests to challenge vasoactivity presenting practical issues due to the amount of effort and cooperation required from subjects5.Relative CVR (rCVR) utilizes resting-state fMRI (rsfMRI) signals to map voxel-wise CVR relative to the global signal5. rsfMRI takes advantage of natural variations in CO2 levels5, similar metric as gas inhalation and breath-holding tests. More recently, whole-brain rCVR has been shown to provide a more feasible approach to quantify brain vessel dilatory capacity with comparable results to conventional whole-brain CVR methodologies5.
NOise Reduction with Distribution Corrected (NORDIC) PCA identifies patterns of signal fluctuations and removes them. The impact of NORDIC-correction can be substantial on rsfMRI, specifically on thermal noise, a zero-mean Gaussian distributed noise6 which originates from the electronics and/or the subject itself7. NORDIC-correction is not expected to affect structured/non-white noise such as spontaneous neuronal activity8. The application of NORDIC-correction to rCVR mapping in both humans and mice, to the best of our knowledge, is yet to be investigated. Here, we evaluated NORDIC-correction on rCVR mapping in humans and mice.
Methods and Materials
Healthy human (n = 21, 41-79 years old) rsfMRI images were collected using a 3-Tesla Siemens scanner and a single-shot EPI pulse sequence with TE/TR=26/2000ms, FOV=20.5×20.5cm2, 64×64 matrix, 38 contiguous 3-mm axial slices, and 240 volumes. Healthy C57BL/6J mouse (n=13, 15-weeks old) rsfMRI images were obtained using a 7-Tesla Bruker scanner and a single-shot EPI pulse sequence with TE/T4=12/1000ms, FOV=16x7mm2, 80x35 matrix, 30 contiguous 0.5-mm axial slices, and 600 volumes. MRICloud (braingps.mricloud.org/rs-cvr) conducted a multi-atlas anatomical segmentation of T1-weighted images, calculated rCVR maps, and extracted rCVR values in human brain regions (Figure 1A).MRICloud calculated rCVR maps for mice (Figure 1B), SPM12 (fil.ion.ucl.ac.uk/spm/) coregistered these rCVR maps, and regions of interest (ROIs) were defined and used to extract rCVR values in the visual cortex (VC), auditory cortex (AC), caudate putamen (CPu), hippocampus (HP), and basal forebrain (BF). NORDIC-correction (github.com/SteenMoeller/NORDIC_Raw) was applied to rsfMRI data prior to MRICloud on the rsfMRI magnitude images. Standard preprocessing were then applied, and tSNR maps were calculated by dividing voxel-wise mean by standard deviation. Results are presented as mean±SEM. Paired t-test, two-way ANOVA, and Levene’s F-test were applied.
Results
NORDIC-correction increases temporal signal-to-noise ratio (tSNR)
NORDIC-correction increases tSNR of the rsfMRI data for both humans (p<0.0001, Figure 2) and mice (p<0.0001, Figure 3). Higher tSNR values may indicate NORDIC-correction can increase the sensitivity of rsfMRI through the removal of noise9.Human rCVR unaffected by NORDIC-correction
Qualitatively, NORDIC-correction did not have an apparent impact on human rCVR values (p=0.2990, Figure 4). The insignificant improvement in human rCVR variance suggests that such variation is dominated by inter-subject variability.NORDIC-correction lowers rCVR variance and potentially improves rCVR along cortical layers in mice
Levene’s F-test showed that NORDIC-correction (σ²: 0.0116) has a significantly lower variance than standard preprocessing (σ²: 0.0144, p=0.0179, Figure 5A). The difference rCVR map between NORIC-correction and standard preprocessing was calculated to assess the difference along the cortical layers (Figure 5B, left). NORDIC-correction increased rCVR along the cortical layers compared to standard preprocessing (p<0.0001, Figure 5B).Discussion
A potential concern to the use of NORDIC-correction on rCVR mapping is how its denoising ability may affect the global signal, and hence affecting rCVR quantification. In this study, the rCVR means between NORDIC-correction and standard preprocessing had no significant difference in both humans (Figure 4) and mice (Figure 5A). Furthermore, NORDIC-correction can lower rCVR variance in mice, indicating higher sensitivity towards rCVR mapping. It is worth noting that the background of the mice in preclinical studies are strictly controlled (e.g., age, genetic makeup, and diet), whereas backgrounds of humans cannot be controlled. Therefore, the variation in humans is most likely dominated by inter-subject variability.The measured rCVR can reflect the conditions of capillaries as they are the primary site of oxygen exchange in the brain. As such, we hypothesize that rCVR is constant along cortical layers as the neocortex consists of a uniform capillary bed10. Our results showed that NORDIC-corrected rCVR was largely constant across cortical layers (Figure 5B), which better reflects the physiology. It is speculated that the lower rCVR at the top-voxel should be due to partial volume effects. Nevertheless, future studies may evaluate NORDIC-correction toward layer-specific rCVR mapping more comprehensively to, and elucidate the characteristics and sources of the removed noise along the cortex relative to the specific transmit/receive coils used. Lastly, the ability of NORDIC-correction to increase tSNR can be considered to trade for higher spatial resolution in brain vascular and functional mapping. This could allow studies to investigate small nuclei in the brain in greater detail.
Conclusion
NORDIC-correction increases tSNR in rsfMRI of both humans and mice, lowers mouse rCVR variance, and potentially improves mouse rCVR along cortical layers. Future rCVR studies investigating cerebrovascular diseases may incorporate NORDIC-correction for higher sensitivity. Overall, these suggest NORDIC-correction may be included as a preprocessing step for rCVR mapping.Acknowledgements
This work was supported in part by the National Institutes of Health P30-CA016087, P41-EB017183, P41-EB027061, R01-EY028125, UO1-EB025144 and UF1-NS107680 (Bethesda, Maryland); BrightFocus Foundation G2019103 (Clarksburg, Maryland); and an unrestricted grant from Research to Prevent Blindness to NYU Langone Health Department of Ophthalmology (New York, New York).References
- Krishnamurthy, V. et al. The Utility of Cerebrovascular Reactivity MRI in Brain Rehabilitation: A Mechanistic Perspective. Front Physiol 12, 642850, doi:10.3389/fphys.2021.642850 (2021).
- Taneja, K. et al. Evaluation of cerebrovascular reserve in patients with cerebrovascular diseases using resting-state MRI: A feasibility study. Magn Reson Imaging 59, 46-52, doi:10.1016/j.mri.2019.03.003 (2019).
- Gupta, A. et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 43, 2884-2891, doi:10.1161/STROKEAHA.112.663716 (2012).
- Catchlove, S. J. et al. Regional Cerebrovascular Reactivity and Cognitive Performance in Healthy Aging. J Exp Neurosci 12, 1179069518785151, doi:10.1177/1179069518785151 (2018).
- Liu, P. et al. Cerebrovascular reactivity mapping using intermittent breath modulation. Neuroimage 215, 116787, doi:10.1016/j.neuroimage.2020.116787 (2020).
- Brooks, J. C., Faull, O. K., Pattinson, K. T. & Jenkinson, M. Physiological noise in brainstem FMRI. Front Hum Neurosci 7, 623, doi:10.3389/fnhum.2013.00623 (2013).
- Moeller, S. et al. NOise reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing. Neuroimage 226, 117539, doi:10.1016/j.neuroimage.2020.117539 (2021).
- Vizioli, L. et al. Lowering the thermal noise barrier in functional brain mapping with magnetic resonance imaging. Nat Commun 12, 5181, doi:10.1038/s41467-021-25431-8 (2021).
- Arturo Cardenas-Blanco, C. T., Pablo Irarrazaval, Ian Cameron. Noise in Magnitude Magnetic Resonance Images. Wiley InterScience 32A(6), 409-416, doi:10.1002/cmr.a.20124 (2008).
- Cipolla, M. J. (2010). The Cerebral Circulation. San Rafael, CA, Morgan & Claypool Life Sciences.
Figures
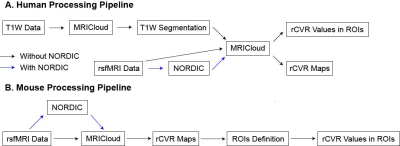
Figure 1: (A) Human processing pipeline used. MRICloud conducted a multi-atlas anatomical segmentation of T1-weighted images, calculated relative cerebrovascular reactivity (rCVR) maps and extracted rCVR values. NORDIC PCA was applied to rsfMRI data prior to MRICloud rCVR calculation. (B) Mouse processing pipeline used. MRICloud was utilized to calculate the rCVR maps, and regions of interest (ROIs) were defined to extract rCVR values. NORDIC PCA was applied to rsfMRI data prior to MRICloud.
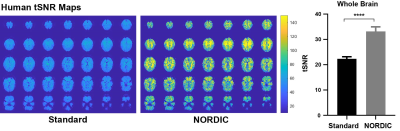
Figure 2: Temporal signal-to-noise ratio (tSNR) is significantly increased with NORDIC-correction across the whole-brain in humans. tSNR maps were calculated in healthy human subjects (n=21, 41-79 years old). The whole-brain tSNR is significantly higher with NORDIC PCA applied (paired t-test, ****p < 0.0001). Error bars indicate +SEM.
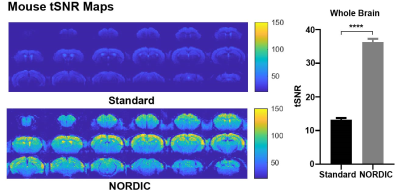
Figure 3: Temporal signal-to-noise ratio (tSNR) is significantly increased with NORDIC-correction across the whole-brain in mice. tSNR maps were calculated in healthy C57BL/6J mice (n=13, 15-weeks old). The whole-brain tSNR is significantly higher with NORDIC PCA applied (paired t-test, ****p < 0.0001). Error bars indicate +SEM.
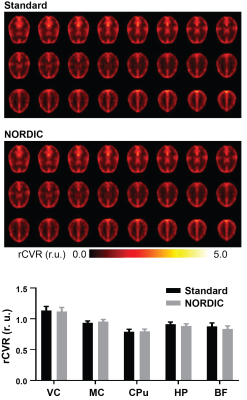
Figure 4: No significant difference was observed between NORDIC-correction and standard preprocessing on human rCVR mapping. Two-way ANOVA was conducted, interaction between regions of interest (ROIs) and preprocessing groups (standard preprocessing vs NORDIC-correction) was not significant (p = 0.5164), nor was between the two preprocessing groups (p = 0.2990). (r.u.: relative unit)
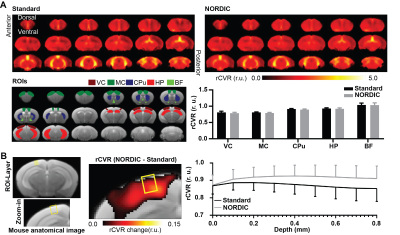
Figure 5: (A) NORDIC-correction decreased mouse rCVR variability. Significant difference observed between the preprocessing groups (standard preprocessing vs NORDIC-correction; Two-way ANOVA, p < 0.05). More importantly, NORDIC-correction decreased mouse rCVR variability (Levene’s F-test, p < 0.05). Error bars indicate +SEM. (B) NORDIC-correction potentially improved mouse rCVR along cortical layers. Significant difference observed between the preprocessing groups (standard preprocessing vs NORDIC-correction; Two-way ANOVA, p < 0.0001). (r.u.: relative unit)
DOI: https://doi.org/10.58530/2022/3326