3317
A Flexible 16-Element Transceiver 8Tx/16Rx Coil Array for Parallel Transmit Cardiac MRI in Pigs at 7T
Ibrahim A. Elabyad1, Maxim Terekhov1, David Lohr1, Maya Bille1, and Laura M. Schreiber1
1Chair of Molecular and Cellular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Wuerzburg, Wuerzburg, Germany
1Chair of Molecular and Cellular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Wuerzburg, Wuerzburg, Germany
Synopsis
A dedicated flexible 16-element transceiver coil array was developed, simulated, and tested for cardiac MRI (cMRI) in pigs at 7T. The 16-elements of the array are printed on a flexible (Kapton polyimide) substrate to conform to the pig thorax. The performance of the elastic array is evaluated by EM-simulations and MR-measurements in a pig phantom and a 45-kg pig in-vivo. The flexible array supports parallel imaging with acceleration factors up to R=4 with reasonable g-factors (g=1.2) in an ROI at the position heart. The array enables geometrical conformity to pig body weights within a large range of sizes and shapes.
Introduction
A wide variety of different coil array designs have allowed significant progress for body imaging at ultrahigh-field (UHF) (e.g., using local Tx/Rx loop1-4 and dipole antenna arrays5,6). Significant advancement of both $$${B_1^+}$$$-shimming and parallel imaging capabilities were demonstrated for in-vivo cMRI in pigs using an antisymmetric 8Tx/16Rx loop array7. To obtain the best image quality for different pigs (25-90kg), three dedicated rigid coil arrays were implemented. These coil arrays have a rigid form and are thus optimized for MR-experiments on pigs with a certain pig weight and thorax shape. However, building an individual coil for each weight range is both time and cost-consuming. The purpose of this work is to design, simulate, implement, and get initial usage experience with elastic coil array, which can be used potentially for cMRI in pigs of a wide range of thorax dimensions and weights (25-90kg).Methods
In this work, we introduce a dedicated transceiver array printed on a flexible 100-µm thick Kapton polyimide (PI) substrate (εr=3.5 and $$$tanδ$$$=0.001). The copper (Cu) trace width is 4mm with 35-µm thickness. The array is composed of 16-elements distributed anti-symmetrically around the central two elements7 [Figure 1]. The sizes of the elements 1, 2, 7, 8, 9, and 10 were 4.5×9.8cm2. The decoupling between the central two elements was accomplished using a common conductor and shared decoupling capacitor (SDC) ($$${C_1^d}$$$). The size of elements 3 and 4 was 5×8cm2. Elements 3 and 4 were decoupled from elements 1 and 2 using SDC ($$${C_2^d}$$$). The size of elements 5 and 6 was 5.6×12.2cm2. The sizes of elements 7 and 9 and the identical elements 8 and 10 are 4.5×9.8cm2. They were decoupled using a shared decoupling capacitor ($$${C_3^d}$$$). Elements 1, 2, and 3 were decoupled from the neighboring elements 5 and 6 using capacitive decoupling ($$${C_5^d}$$$, $$${C_6^d}$$$, and $$${C_7^d}$$$) in addition to a decoupling gap of 2cm. The external dimension for the array was 57.9×20.8cm2. EM-simulations were performed using CST-Microwave-Studio for coil design and $$${B_1^+}$$$-field optimizations. For matching, tuning, and decoupling, RF-circuit co-simulation was employed in CST-Design-Studio to get an initial guess for the optimal lumped elements8. Static phase-only $$${B_1^+}$$$-shimming was performed within a pig phantom using two different optimization cost functions ($$${FC_1}$$$ for $$${B_1^+}$$$-homogeneity and $$${FC_2}$$$ for weighted combination of $$${B_1^+}$$$-homogeneity and transmit efficiency7. To characterize the Tx performance of the array, $$$CoV=\frac{std(B_1^+)}{mean(B_1^+)}$$$ and $$$Tx_{eff}=\frac{mean(B_1^+)}{\sqrt{P_{acc}}}$$$ were computed. To form an 8Tx/16Rx array compatible with the pTx system, every two neighboring elements were combined in one Tx-channel [Figure 1a]. All measurements were performed on a 7T whole-body MAGNETOM Siemens Terra scanner. The array was tested in phantom with the measurement of SNR-maps, $$${B_1}$$$-shimming, g-factor with high parallel imaging acceleration factors. The reconstructed experimental g-factor and SNR-maps were post-processed in MATLAB. Female pigs (German Landrace) were used after approval by the responsible local animal welfare committee (project 55.2 DMS 2532-1134-16, Regierung von Unterfranken). All in-vivo measurements were done using a GRE cardiac sequence (BEAT) provided by the scanner vendor. To visualize RF-excitation coverage of the heart volume, 10 coronal, sagittal, and transversal slices positioned within the heart were acquired with prospective triggering. Imaging parameters were: TR=44ms, TE=2.9ms, matrix size=150x120, FOV=300x300mm2, slice thickness 6mm, inter-slice distance 1.2mm, FA=20°. Then CINE-imaging with a retrospective reconstruction of 30 heart phases was performed for standard cardiologic views of the heart: four-chamber, two-chamber, and SA.Results
Figure 2 shows the simulated $$${B_1^+}$$$-field distribution for the 16-element flexible array after static phase-only $$${B_1^+}$$$-shimming within a dedicated pig phantom with PV1 and PV2 compared to the zero phases. With PV1, $$$CoV$$$ was improved by 70% in the central transversal slice compared to zero-phases. With PV2, $$$Tx_{eff}$$$ was improved by 3-times compared to zero-phases. Figure 3 shows the measured g-factor maps (using acceleration factors R=2, 3, 4, and 6), the SNR-maps, the noise correlation matrix of the flexible array loaded with a pig phantom, and in-vivo pig. The mean SNR within the heart region from the novel design was 70. With the implemented hardware phases and even prior $$${B_1^+}$$$-shimming, the coil shows good Rx properties with high SNR. Figures 4 and 5 show in-vivo images of a 45-kg pig with normal and high resolutions acquired using the flexible array. The developed coil demonstrates sufficient $$${B_1^+}$$$-field homogeneity within the pig heart without significant destructive interference.Discussion
The initial experience of using the coil in the animal study has demonstrated that the fully elastic array provides sufficient $$$Tx_{eff}$$$ with $$${B_1^+}$$$-penetration on the full depth of the pig heart and coverage of the whole heart volume. The reasonable imaging quality with SNR moderately lower than delivered by the dedicated rigid array optimized for specific weight range was shown. Further optimizations of the tuning/matching circuits should be performed to make possible application of the fully elastic array for the wide range of the animal’s thorax shapes.Conclusion
A dedicated flexible pig array with the best bendability to conform to the thorax shape of several pigs was introduced. All fixed capacitors, cable traps, and semi-rigid cables were fixed on the flexible PI-substrate without getting broken from several bending. Further optimizations of the tuning/matching will be performed to optimize the array for a wide range of pig weights.Acknowledgements
This project was funded by the Federal Ministry of Education and Research (BMBF), Grant/Award Number: 01EO1004 & 01EO1504.References
- Graessl A, Renz W, Hezel F, et al. Modular 32-channel transceiver coil array for cardiac MRI at 7.0T. Magn Reson Med. 2014;72(1):276-290.
- Dieringer MA, Renz W, Lindel T, et al. Design and application of a four-channel transmit/receive surface coil for functional cardiac imaging at 7T. J Magn Reson Imaging. 2011;33(3):736-741.
- Grassl A, Winter L, Thalhammer C, et al. Design, evaluation and application of an eight channel transmit/receive coil array for cardiac MRI at 7.0 T. Eur J Radiol. 2013;82(5):752-759.
- Thalhammer C, Renz W, Winter L, et al. Two-dimensional sixteen channel transmit/receive coil array for cardiac MRI at 7.0 T: design, evaluation, and application. J Magn Reson Imaging. 2012;36(4):847-857.
- Raaijmakers AJ, Ipek O, Klomp DW, et al. Design of a radiative surface coil array element at 7 T: the single-side adapted dipole antenna. Magn Reson Med. 2011;66(5):1488-1497.
- Raaijmakers AJ, Italiaander M, Voogt IJ, et al. The fractionated dipole antenna: A new antenna for body imaging at 7 Tesla. Magn Reson Med. 2016;75(3):1366-1374.
- Elabyad IA, Terekhov M, Lohr D, Stefanescu MR, Baltes S, Schreiber LM. A Novel Mono-surface Antisymmetric 8Tx/16Rx Coil Array for Parallel Transmit Cardiac MRI in Pigs at 7T. Scientific Reports. 2020;10(1):3117.
- Kozlov M, Turner R. Fast MRI coil analysis based on 3-D electromagnetic and RF circuit co-simulation. Journal of Magnetic Resonance. 2009;200(1):147-152.
Figures
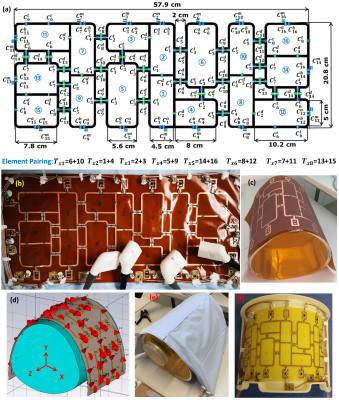
Figure
1 (a)
Schematic of the 16-element antisymmetric flexible array with dimensions, element number, and element pairing: m denotes for matching, t for tuning, d for decoupling, and a, b for splitting tuning. Every two neighbouring loops are
paired to form an 8Tx/16Rx array compatible with the pTx system. (b) A
prototype of the new 16-element antisymmetric flexible array. (c) The PCB of
the array on the pig phantom. (d) RF simulation model. (e) A
prototype of the flexible array loaded with a pig phantom. (f) A prototype of the
reference 16-element antisymmetric rigid 8Tx/16Rx array7.
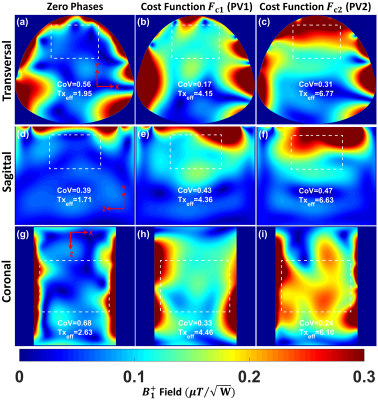
Figure
2 Simulated
combined $$${B_1^+}$$$ field
distribution in central transversal, sagittal, and coronal planes
after static phase-only $$${B_1^+}$$$ shimming for the flexible
array loaded within a pig phantom using two different $$${B_1^+}$$$ optimization
cost functions ($$${FC_1}$$$ and $$${FC_2}$$$) compared to zero phases. The array was fed with 1W accepted power and all coil
elements were fed with equal amplitudes. The $$$CoV$$$ and $$$Tx_{eff}$$$ in $$$\frac{\mu{T}}{\sqrt{kW} }$$$ were computed
in the selected ROIs.
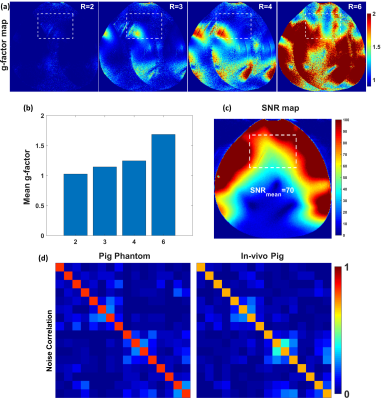
Figure
3 (a) Measured
g-factor maps of R=2, 3, 4, and 6 within a pig phantom with acceleration in the
L−R direction were acquired using the flexible array. (b) Mean values of the
g-factors were evaluated in the selected ROI of the heart. (c) SNR maps. (d) Measured noise correlation matrix of the flexible
array loaded with a pig phantom and in-vivo
pig.
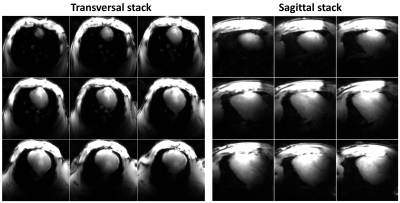
Figure
4 Stack
of transversal (left) and sagittal (right) slices of in-vivo images of the pig heart
acquired with elastic array prototype. Sufficient $$${B_1^+}$$$ penetration
depth with coverage of both lateral and posterior walls of the heart is
confirmed.
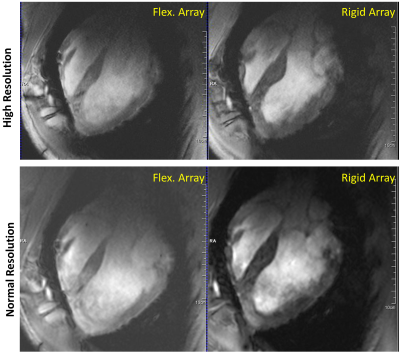
Figure
5 In-vivo long-axis
heart view CINE images of a 45 kg pig with normal (1.5x1.5mm/pixel in-plane)
and increased (1.2x1.2mm pixel) spatial resolution acquired using the elastic
flexible and rigid arrays, respectively.
DOI: https://doi.org/10.58530/2022/3317