3313
Direct Signal Control combined with dynamic, Fast Online Customized (FOCUS) parallel transmit excitation pulses for TSE at 7 Tesla1Department of Neuroradiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2MR Research Collaborations, Siemens Healthcare Limited, London, United Kingdom, 3Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 4Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom, 5Siemens Healthcare GmbH, Erlangen, Germany, 6Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 7Department of Computer Science, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 8Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
Direct Signal Control with Variable Excitation and Refocusing (DiSCoVER) was combined with a dynamic, Fast Online-Customized (FOCUS) parallel transmit excitation pulse for a 3D TSE sequence using an 8Tx/32Rx head RF coil at 7 Tesla. DSC could improve the signal, mainly in the cerebellum region compared to CP mode. FOCUS excitation pulses achieve better FA and phase homogeneity than static pTx pulses (standardly used in DiSCoVER). Combining a FOCUS excitation pulse with DiSCoVER-optimized refocusing pulses, led to signal gain across the echoes, which was evaluated in simulations for 132 subjects. Universal and individual DiSCoVER-refocusing pulses showed comparable performance.
Introduction
Turbo-spin echo (TSE) sequences at ultra-high fields suffer from poor B1+ homogeneity and conservative local specific absorption rate (SAR) limits1. Direct signal control with Variable Excitation and Refocusing (DiSCoVER), using parallel transmit (pTx) pulses, has shown to improve the MR signal homogeneity across a refocusing pulse train under strict SAR constraints2. This method calculates individually optimized, pTx scale factors for each pulse in the train (excitation and refocusing) and has been integrated into an MR system despite requiring to solve a large problem during the scan (one minute online-calculation3). Dynamic pTx pulses apply time-varying B1+-fields and apply B0-field gradients to adjust the nuclear spin phases during the pulse. These have shown greater potential to achieve homogeneous flip angle (FA) and phase patterns than static pTx4. Universal pulses (UPs), as exemplary dynamic pTx pulses, have been shown to be applicable for both gradient echo (GRE)5 and TSE6 sequences with robust performance and without the need of any online-calculation time. To individually optimize dynamic pTx pulses in a clinically acceptable time (~1 minute), fast online-customized (FOCUS) pulses have been presented, which consist of a universal pulse and parameter optimization prior to the scan and a thereby faster and more robust individual online-optimization7. In this work, we combine a dynamic, FOCUS pTx excitation pulse with refocusing pulses that are optimized with DiSCoVER for a 3D TSE sequence using an 8Tx/32Rx head coil (Nova Medical, Wilmington, USA) at 7 Tesla.Methods
Firstly, a nonselective, dynamic, universal pTx excitation pulse was optimized by using a kT point trajectory with globally optimizing 6 kT point locations and respective sub-pulse durations in one step and, in a second step, corresponding universal pulse shapes. We used B1+ and B0 maps of 20 training subjects and a specifically optimized interior-point method for the optimization8. The UP served as an initialization for further individual optimization to achieve a 90° FA and homogeneous phase excitation, which was assumed to provide optimal conditions for generating TSEs with high signal. Bloch simulations were performed for the FOCUS excitation pulses, applied on every subject to obtain the corresponding FA and phase patterns.Secondly, based on these patterns, the following 9 static pTx refocusing pulses were optimized universally across all training subjects by using DiSCoVER. Then, further individually DiSCoVER-optimized refocusing pulses were generated, using the universal ones as initialization.
Finally, four different cases were evaluated in simulations and compared with respect to their achieved homogeneity under the same SAR (channel power limits) and peak-voltage constraints: Purely circularly polarized (CP) pulses (1), DiSCoVER (2) and a FOCUS, dynamic excitation pulse, followed by refocusing pulses, which were optimized either universally (3) or individually (4) using DiSCoVER.
Results
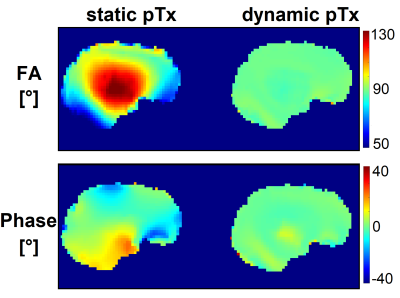
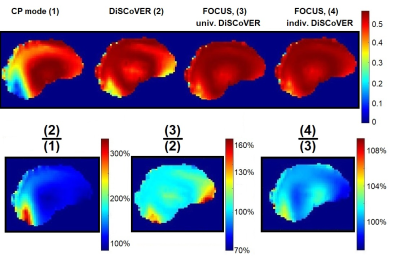
Figure 1 shows the FA and phase distribution in a central sagittal brain slice of one subject for a static and a FOCUS, dynamic pTx excitation pulse. Both distributions become clearly more homogeneous when using the dynamic pulse.Figure 2 shows the simulated spatial signal distribution, relative to the target signal (starting from 1, relaxation with T1=1s and T2=70ms in every voxel) in a central sagittal slice of another subject, averaged over the first 10 echoes for the four mentioned cases. DiSCoVER reaches much stronger signal compared to the CP mode, especially in the cerebellum. Further improvement is reached when using a dynamic excitation pulse and universal DiSCoVER-calculated shims. The highest signal gain is achieved in the frontal lobe and cerebellum. Individually optimized shims then only slightly change the signal, compared to universal ones.
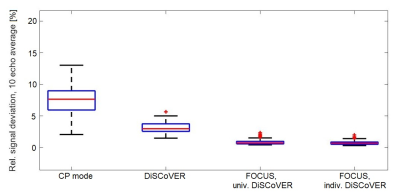
The relative differences of these echo-averaged signals from the target were then evaluated on 132 subjects and averaged over all valid voxels across the brain region. The resulting scalar values are shown in Figure 3. It demonstrates lower signal deviations in both mean and variance among different subjects when using DiSCoVER instead of CP mode and also when using FOCUS excitation pulses instead of standard ones across all subjects.
Discussion and Conclusions
DiSCoVER can be further improved when combined with a dynamic, FOCUS pTx excitation pulse. The FOCUS pulse achieves more homogeneous FA and phase patterns than a static pTx pulse. Additionally, an individual DiSCoVER-optimization shows similar quality as previously universally optimized refocusing pulses. Thus, as also suggested to earlier study findings9, skipping the DiSCoVER-optimization time of approximately one minute3 could ultimately be a valid option. In this case, after rapid B1+ and B0 mapping, only about 6 seconds online-optimization time for the FOCUS excitation pulse would be necessary.To further improve the performances, the dynamic excitation pulses could be integrated into the DiSCoVER-optimization. Additionally, the refocusing pulses could consist of several sub-pulses (B1+-fields) and gradient fields as well, which might lead to even better homogeneity throughout the echo train. On the other hand, this would require an even more complex problem to solve with DiSCoVER. Furthermore, it might lead to worse SAR efficiency when using fixed per-channel power limits. To still guarantee safety and meet the local SAR limits, virtual observation points (VOPs)10, which estimate spatial distribution of local SAR exposure for different B1+ fields, could be advantageous. Those have shown a potentially great reduction of the local SAR estimations, especially for highly dynamic pulses (many different B1+ fields)11.
Acknowledgements
The authors thank Dr. Ioannis-Angelos Giapitzakis for proofreading and useful suggestions that helped to improve this abstract.References
1 Fiedler, T. M., Ladd, M. E. & Bitz, A. K. SAR Simulations & Safety. Neuroimage 168, 33-58, doi:10.1016/j.neuroimage.2017.03.035 (2018).
2 Malik, S. J., Beqiri, A., Padormo, F. & Hajnal, J. V. Direct signal control of the steady-state response of 3D-FSE sequences. Magn Reson Med 73, 951-963, doi:10.1002/mrm.25192 (2015).
3 Tomi-Tricot, R. et al. Fully Integrated Scanner Implementation of Direct Signal Control for 2D T2-Weighted TSE at Ultra-High Field. 29th Annual Meeting of the Intl. Soc. Magn. Reson. Med. (2021).
4 Padormo, F., Beqiri, A., Hajnal, J. V. & Malik, S. J. Parallel transmission for ultrahigh-field imaging. Nmr in Biomedicine 29, 1145-1161, doi:10.1002/nbm.3313 (2016).
5 Gras, V., Vignaud, A., Amadon, A., Le Bihan, D. & Boulant, N. Universal pulses: A new concept for calibration-free parallel transmission. Magn Reson Med 77, 635-643, doi:10.1002/mrm.26148 (2017).
6 Gras, V. et al. Robust nonadiabatic T2 preparation using universal parallel-transmit kT -point pulses for 3D FLAIR imaging at 7 T. Magn Reson Med 81, 3202-3208, doi:10.1002/mrm.27645 (2019).
7 Herrler, J. et al. Fast online-customized (FOCUS) parallel transmission pulses: A combination of universal pulses and individual optimization. Magn Reson Med 85, 3140-3153, doi:10.1002/mrm.28643 (2021).
8 Majewski, K. Simultaneous optimization of radio frequency and gradient waveforms with exact Hessians and slew rate constraints applied to kT-points excitation. J Magn Reson 326, 106941, doi:10.1016/j.jmr.2021.106941 (2021).
9 Beqiri, A., Hoogduin, H., Sbrizzi, A., Hajnal, J. V. & Malik, S. J. Whole-brain 3D FLAIR at 7T using direct signal control. Magn Reson Med 80, 1533-1545, doi:10.1002/mrm.27149 (2018).
10 Eichfelder, G. & Gebhardt, M. Local Specific Absorption Rate Control for Parallel Transmission by Virtual Observation Points. Magnetic Resonance in Medicine 66, 1468-1476, doi:10.1002/mrm.22927 (2011).
11 Williams, S. N. et al. SAR Management in pTx Sequence Design: The Impact of Electromagnetic-Field-Derived Virtual Observation Points. 29th Annual Meeting of the Intl. Soc. Magn. Reson. Med. (2021).
Figures