3312
Neural Network-supported Fast Online-Customized (FOCUS) parallel transmit (pTx) pulses for slice-selective, large flip angle excitation1Department of Neuroradiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Department of Corporate Technology, Siemens, München, Germany, 4Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 5Department of Computer Science, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 6Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
Slice-selective, two-spoke parallel transmit (pTx) pulses for exciting large flip angles can be designed quickly and show robust performance for MRI at 7 Tesla using an 8Tx/32Rx coil. Firstly, clusters of B1+/B0 maps are defined and corresponding pulses are optimized prior to the scan. These serve as initialization for a fast, gradient-descent based online-optimization. For each slice, the best cluster-specific initialization is chosen online via neural networks, which predict their respective spatial distribution of the longitudinal magnetization. This approach outperforms other strategies to initialize the pTx RF pulses such as offline-optimized slab-specific and circularly polarized RF pulses as initializations.
Introduction
Poor homogeneity of the transmit (B1+) fields in MRI at 7 Tesla may cause regions of low signal intensity and contrast variations1. Dynamic parallel transmit (pTx) pulses have shown great potential to overcome these limitations and produce uniform flip angle (FA) distributions but are rarely used in a clinical setting due to the complexity and non-convexity of the pulse design problem2. The concept of Universal Pulses (UPs) has been proposed and proven to be robust and clinically applicable for a large variety of applications and body parts3-5. It could also be extended to cases with larger inter-subject variability using machine learning6. Furthermore, nonselective Fast Online-Customized (FOCUS) pulses have been proposed for small7 and large8 FA excitations. This method consists of optimizing universal pulses and parameter values offline, which then enable a robust and quicker individual online-optimization.In this work, we show that the FOCUS-concept can be extended to slice-selective excitation of large FAs despite of largely varying field patterns. We use the well-established spokes-pulses9, which, however, provide only few degrees of freedom and may produce signal dropouts in some cases that currently can only be avoided for small FAs with a specifically developed regularization10. In this work, we generate clusters of field-maps (all channels’ B1+ and B0 maps) and corresponding UPs. Then we train one neural network (NN) for each cluster’s UP to predict the longitudinal magnetization (Mz) on every slice, when it is used as initialization for further individual optimization. Using these NNs, we choose the best possible initialization among the three cluster-specific pulses. The shown simulations were based on field-maps acquired with a saturation-prepared turbo FLASH sequence using an 8Tx/32Rx RF coil (Nova Medical, Wilmington, USA) at 7 Tesla.
Methods
In general, all pulses consisted of 2 spokes and were optimized using an interior-point method optimization using exact Hessians to simultaneously optimize the RF shapes and spoke locations11 for a target FA of 80°.Clustering
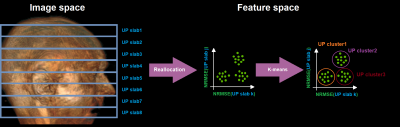
This process is illustrated in Figure 1. As a first step, all transversal slices of in total 20 subjects were interpolated into a universal 3D field of view (FOV). It consisted of 8 universal slab positions with a slab thickness of 28.6 mm and 4 mm in-plane resolution. All subject’s slices in each slab were used to optimize a slab-specific UP. In the next step, all slab-specific pulses were applied on each slice in the dataset. Their resulting FA-NRMSEs were then used to perform a k-means clustering12. Based on the decrease of the within-cluster sum of squares (WCSS) value for ascending cluster numbers, we chose K=3 clusters. From these 3 clusters, we took the 10 slices with the minimum Euclidean distance to the respective centroid to optimize a cluster-specific UP.
Neural Network training
For each cluster, a NN was trained with the normalized B1+ and B0 maps of 132 subjects, interpolated into the universal FOV’s x- and y-dimensions. These predict the normalized spatial distribution of Mz(r) = cos(FA(r)) after an individually optimized pulse using this cluster’s specific UP as initialization. The NNs were based on a u-net, originally used for tumor segmentation (https://www.kaggle.com/monkira/brain-mri-segmentation-using-unet-keras), with slight architecture-modifications and using the magnitude squared error (MSE) as loss. 4173 transversal slices were used for training, 1044 for validation and 580 for final testing.
Overall Pulse Design Process
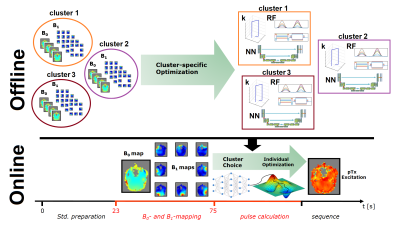
Figure 2 shows the general process of the NN-supported FOCUS pulse design. Prior to the scan, 3 cluster-specific pulses were generated. During the scan, B1+ and B0 maps were acquired and served as input for the NNs to predict the expected NRMSE when using the different cluster-specific pulses as initialization. Thereby, one of the cluster-specific pulses is chosen as starting point for the individual, interior-point method optimization11.
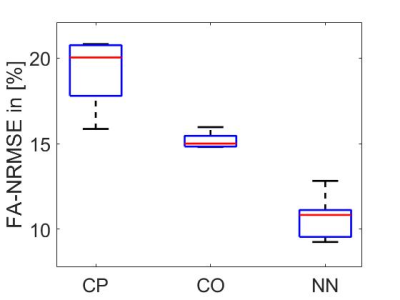
PTx pulses were evaluated using three different initialization-strategies for the individual online-optimization under the same local specific absorption rate (SAR) and peak-voltage constraint: Circularly polarized (CP) pulses and both spokes located in the center (1), coordinate (CO)-based choice of the 8 slab-specific UPs mentioned above (2) and NN-based choice of 3 cluster-specific UPs (3).
Results
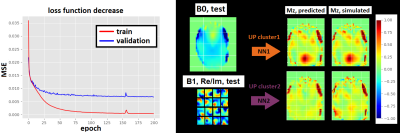
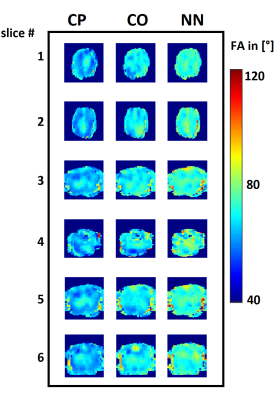
Figure 3 shows the loss function decrease for different numbers of epochs during the training of one NN, as well as predictions of Mz for two NNs and corresponding UP-initializations in one slice. These have shown to be accurate enough to prevent cases with severely low FAs by choosing a better suitable cluster-specific UP.Figure 4 shows simulated FA maps of 6 randomly chosen, exemplary slices of 8 test subjects using the three mentioned initialization strategies. Figure 5 illustrates the normalized root mean squared error (NRMSE) of the FA of the same test subjects, averaged over all slices. The NN-based initialization strategy shows the best homogeneity, followed by CO-based initializations, which in turn outperforms purely CP initializations.
Discussion and Conclusions
NN-supported FOCUS pulses with cluster-specific offline-optimizations robustly achieve homogeneous, slice-selective, large FA excitations in the head. Yet, strong B0 offsets lead to bad performances regardless of the initialization. To further improve those, the sinc-shaped RF sub-pulses could be replaced by ones that provide more ΔB0-robust excitation patterns in preferably short online-optimization time (e.g. DeepControl13). In general, this approach can be transferred to other applications and body parts with high inter-subject variability.Acknowledgements
The authors thank Dr. Ioannis-Angelos Giapitzakis for proofreading and useful suggestions, which helped to improve this abstract.References
1 Ladd, M. E. et al. Pros and cons of ultra-high-field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc 109, 1-50, doi:10.1016/j.pnmrs.2018.06.001 (2018).
2 Padormo, F., Beqiri, A., Hajnal, J. V. & Malik, S. J. Parallel transmission for ultrahigh-field imaging. Nmr in Biomedicine 29, 1145-1161, doi:10.1002/nbm.3313 (2016).
3 Gras, V., Vignaud, A., Amadon, A., Le Bihan, D. & Boulant, N. Universal pulses: A new concept for calibration-free parallel transmission. Magn Reson Med 77, 635-643, doi:10.1002/mrm.26148 (2017). 4 Aigner, C. S., Dietrich, S., Schaeffter, T. & Schmitter, S. Calibration-free pTx of the human heart at 7T via 3D universal pulses. Magn Reson Med, doi:10.1002/mrm.28952 (2021).
5 Gras, V. et al. Homogeneous non-selective and slice-selective parallel-transmit excitations at 7 Tesla with universal pulses: A validation study on two commercial RF coils. PLoS One 12, e0183562, doi:10.1371/journal.pone.0183562 (2017).
6 Tomi-Tricot, R. et al. SmartPulse, a machine learning approach for calibration-free dynamic RF shimming: Preliminary study in a clinical environment. Magn Reson Med 82, 2016-2031, doi:10.1002/mrm.27870 (2019).
7 Herrler, J. et al. Fast online-customized (FOCUS) parallel transmission pulses: A combination of universal pulses and individual optimization. Magn Reson Med 85, 3140-3153, doi:10.1002/mrm.28643 (2021).
8 Herrler, J. et al. Evaluating Universal and Fast Online Customized Pulses for parallel transmission using two different RF coils in 29th Annual Meeting of the Int. Soc. Magn. Reson. Med.
9 Saekho, S., Yip, C. Y., Noll, D. C., Boada, F. E. & Stenger, V. A. Fast-kz three-dimensional tailored radiofrequency pulse for reduced B1 inhomogeneity. Magn Reson Med 55, 719-724, doi:10.1002/mrm.20840 (2006).
10 Paez, A., Gu, C. & Cao, Z. Robust RF shimming and small-tip-angle multispoke pulse design with finite-difference regularization. Magn Reson Med 86, 1472-1481, doi:10.1002/mrm.28820 (2021).
11 Majewski, K. Simultaneous optimization of radio frequency and gradient waveforms with exact Hessians and slew rate constraints applied to kT-points excitation. J Magn Reson 326, 106941, doi:10.1016/j.jmr.2021.106941 (2021).
12 Lloyd, S. Least squares quantization in PCM. IEEE Transactions on Information Theory 28, 129-137, doi:10.1109/TIT.1982.1056489 (1982).
13 Vinding, M. S., Aigner, C. S., Schmitter, S. & Lund, T. E. DeepControl: 2DRF pulses facilitating B 1 + inhomogeneity and B0 off-resonance compensation in vivo at 7 T. Magn Reson Med 85, 3308-3317, doi:10.1002/mrm.28667 (2021).
Figures