3307
In vivo validation and quantification of non-susceptibility frequency contrast obtained with DEEPOLE QUASAR1Department of Computer Science and Automation, Technische Universität Ilmenau, Ilmenau, Germany, 2Buffalo Neuroimaging Analysis Center, Department of Neurology at the Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, NY, United States, 3Center for Biomedical Imaging, Clinical and Translational Science Institute, University at Buffalo, The State University of New York, Buffalo, NY, United States
Synopsis
Sources of MRI phase contrast include magnetic susceptibility, chemical exchange, and tissue microstructure. The novel DEEPOLE QUASAR method disentangles frequency sources and yields separate maps for magnetic susceptibility and non-susceptibility frequency shifts.
In this work, we validated the method in vivo and performed the first quantitative study of non-susceptibility frequency in the human brain. We found substantial non-susceptibility contributions in WM and GM. The quantification of non-susceptibility in the human brain provides new ground for theories on the origins of frequency contrast.
Introduction
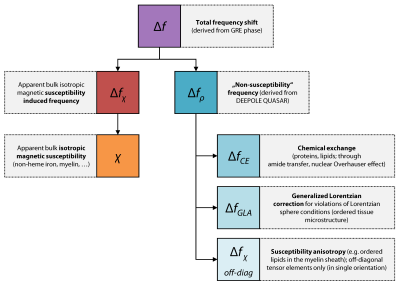
Frequency maps, derived from gradient-echo MRI phase data, reveal local shifts of the Larmor frequency in brain tissue.1 These maps have been used directly for imaging anatomical contrast2 and as a basis for the quantitative assessment of magnetic tissue properties.3 The quantitative estimates from frequencies are delicate, because the recorded voxel-average frequency shift is the result of multiple intricately overlapping mechanisms. Current approaches to translate frequency maps into quantitative tissue properties involve approximations with dire consequences. The widely-used quantitative susceptibility mapping (QSM), for example, requires isotropic magnetic susceptibility to be the exclusive source of frequency shift.3 Furthermore, QSM implicitly assumes that the Lorentzian approach4 with a spherical cavity5 may be used, which requires isotropic or randomly oriented susceptibility inclusions in the tissue—a condition that has been disputed for white matter brain tissue with its strong molecular order around the aligned axons.3 Besides the bulk isotropic magnetic susceptibility considered in the QSM model, other frequency contributions have been predicted and experimentally demonstrated in brain tissues, including (i) chemical exchange,6,7 (ii) susceptibility anisotropy,8,9 and (iii) anisotropic microstructural compartmentalization.10,11 The simplistic biophysical model of QSM not only introduces errors to the susceptibility estimates,8 it also neglects the additional information about these remaining “non-susceptibility” frequency sources and its potential clinical applications.12Mapping non-susceptibility might help us to advance our understanding of the sources of frequency contrast in the healthy and diseased brain. In white matter, the non-susceptibility has been predicted to be an early marker of myelin disintegration that could help to tell apart different types of tissue alteration in multiple sclerosis lesions.13 For its quantitative nature, it can reveal diffuse tissue alterations and might be translated into tissue properties today only accessible via biopsy.14
Recently, the novel DEEPOLE QUASAR15,16 method was introduced, which can disentangle frequency contrast into two separate sources: the isotropic magnetic susceptibility χ and the non-susceptibility frequency shifts fρ. DEEPOLE QUASAR solves the inverse problem posed by the QUASAR model12,
$$f=d*\chi+f_{\rho}$$
where d represents the unit magnetic dipole response with Lorentzian sphere correction and * denotes the spatial convolution.
In this work, we validate the method in vivo, present the first quantitative results from mapping non-susceptibility frequency in the human brain, and discuss potential origins of the observed frequency contrast.
Methods
Susceptibility and non-susceptibility frequency mapping: We used DEEPOLE QUASAR15,16 to approximate the inverse mappings from f to χ and fρ, respectively (Fig. 1A).Validation: We compared the reconstruction performance of DEEPOLE QUASAR to conventional QSM in 120 synthetic volumes with known ground truth. To validate DEEPOLE QUASAR in vivo, we applied chemical exchange and susceptibility tensor imaging (ChEST),17 an extended version of susceptibility tensor imaging that includes a rotation-invariant term.18 From multiple orientations, ChEST estimates maps of mean magnetic susceptibility χ, magnetic susceptibility anisotropy MSA, and a local rotation-invariant offset fCE, resumably related to chemical exchange. In the QUASAR model, fρ includes fCE as well as the anisotropy-induced frequency fMSA shift related to off-diagonal susceptibility tensor elements because neither of those present with the characteristic dipole field pattern of isotropic susceptibility. We used the 12-orientation dataset (3T) by Li et al.19
Human study: In data from 12 volunteers (12 echoes, 3T, axial 3D multi-echo spoiled GRE), we quantified susceptibility and non-susceptibility frequency in cerebrospinal fluid (CSF), white matter (WM), and several gray matter (GM) regions. Deep gray matter and lateral ventricle regions were drawn on a template brain and multimodally registered, based on T1-weighted images and susceptibility maps. WM, CSF and a total GM region were automatically segmented (MRtrix 5ttgen20) and a cortical gray matter region was derived by removing the atlas-based deep gray matter regions from the automatically segmented full gray matter region. The lateral ventricles were used as the zero-reference.
Results and Discussion
The in silico results (Fig. 1B) demonstrated DEEPOLE QUASAR’s ability to accurately and robustly reconstruct χ and fρ from a superposition of d*χ and fρ. Normalized RMSE(χDEEPOLE)=0.39 < normalized RMSE(χQSM)=1.40, N=120 volumes.The comparison with ChEST (Fig. 2) showed a strong similarity between fρ (from a single orientation), and the superposition of chemical exchange-induced frequency with susceptibility anisotropy-related frequency (from 12 orientations).
Fig. 3 shows maps of χ and fρ from a healthy subject and Table 1 summarizes the ROI results. We observed significant fρ contributions in most ROIs, confirming Wharton’s prediction for WM.8 Contrary to Shmueli’s observation for fCE at 14T,6 fρ in WM was lower than in cortical GM (-3.50±1.37 ppb) and higher than in CSF (6.75±3.99) (conflicting with the “white matter darkness” theory13).
Fig. 4 provides an overview of the experimentally shown and/or predicted generators of frequency contrast in brain tissue and which computed maps they are attributed to.
Conclusion
We performed the first quantitative study of non-susceptibility frequency in the human brain and found substantial non-susceptibility contributions in WM and GM. In our validation experiments, DEEPOLE QUASAR’s susceptibility estimates were more robust against non-susceptibility frequency perturbations than those from QSM. Since susceptibility values are increasingly being interpreted in clinical studies, DEEPOLE QUASAR should be considered and further validated as an alternative to QSM.Our quantification of non-susceptibility in the human brain provides new ground for theories on the origins of frequency contrast.
Acknowledgements
The local Ethical Standards Committee approved the human experiments, and a written informed consent form was obtained.We thank Xu Li (Johns Hopkins University, USA) for granting access to the 12-orientation human dataset, and Hwihun Jeong and Jongho Lee (Seoul National University, Korea) for providing the corresponding ChEST solutions.
Research reported in this publication was partially supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS114227 (F.S.) and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001412 (F.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Furthermore, the research was supported by the German Federal Ministry of Education and Research (BMBF) grant TeleBrain (01DS19009A), the Free State of Thuringia within the ThiMEDOP project (2018 IZN 0004) with funds of the European Union (EFRE), and the Free State of Thuringia within the thurAI project (2021 FGI 0008).
References
1. Young, I. et al. Clinical magnetic susceptibility mapping of the brain. Journal of computer assisted tomography 11, 2–6 (1987).
2. Duyn, J. H. et al. High-field MRI of brain cortical substructure based on signal phase. Proceedings of the National Academy of Sciences 104, 11796–11801 (2007).
3. Schweser, F., Deistung, A. & Reichenbach, J. R. Foundations of MRI phase imaging and processing for Quantitative Susceptibility Mapping (QSM). Zeitschrift für Medizinische Physik 26, 6–34 (2016).
4. Lorentz, H. A. The theory of electrons and its applications to the phenomena of light and radiant heat. (Leipzig : B.G. Teubner ; New York : G.E. Stechert, 1916).
5. Marques, J. P. & Bowtell, R. Application of a Fourier‐based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts in Magnetic Resonance Part B: Magnetic Resonance Engineering 25B, 65–78 (2005).
6. Shmueli, K., Dodd, S. J., Li, T.-Q. & Duyn, J. H. The contribution of chemical exchange to MRI frequency shifts in brain tissue. Magnetic Resonance in Medicine 65, 35–43 (2011).
7. Leutritz, T., Hilfert, L., Smalla, K.-H., Speck, O. & Zhong, K. Accurate quantification of water–macromolecule exchange induced frequency shift: Effects of reference substance. Magnetic Resonance in Medicine 69, 263–268 (2013).
8. Wharton, S. & Bowtell, R. Effects of white matter microstructure on phase and susceptibility maps. Magnetic Resonance in Medicine 73, 1258–1269 (2015).
9. Lee, J. et al. Sensitivity of MRI resonance frequency to the orientation of brain tissue microstructure. Proceedings of the National Academy of Sciences 107, 5130–5135 (2010).
10. He, X. & Yablonskiy, D. A. Biophysical mechanisms of phase contrast in gradient echo MRI. Proceedings of the National Academy of Sciences 106, 13558–13563 (2009).
11. Wharton, S. & Bowtell, R. Fiber orientation-dependent white matter contrast in gradient echo MRI. PNAS 109, 18559–18564 (2012).
12. Schweser, F. & Zivadinov, R. Quantitative susceptibility mapping (QSM) with an extended physical model for MRI frequency contrast in the brain: a proof‐of‐concept of quantitative susceptibility and residual (QUASAR) mapping. NMR in Biomedicine (2018) doi:10.1002/nbm.3999.
13. Yablonskiy, D. A., Luo, J., Sukstanskii, A. L., Iyer, A. & Cross, A. H. Biophysical mechanisms of MRI signal frequency contrast in multiple sclerosis. Proceedings of the National Academy of Sciences 109, 14212–14217 (2012).
14. Weiskopf, N., Edwards, L. J., Helms, G., Mohammadi, S. & Kirilina, E. Quantitative magnetic resonance imaging of brain anatomy and in vivo histology. Nat Rev Phys 1–19 (2021) doi:10.1038/s42254-021-00326-1.
15. Jochmann, T., Haueisen, J., Zivadinov, R. & Schweser, F. U2-Net for DEEPOLE QUASAR–A Physics-Informed Deep Convolutional Neural Network that Disentangles MRI Phase Contrast Mechanisms. (2019).
16. Jochmann, T. et al. Quantitative mapping of susceptibility and non-susceptibility frequency with DEEPOLE QUASAR. in International Society of Magnetic Resonance in Medicine (ISMRM) 29th Annual Meeting (2021).
17. Jeong, H., Shin, H.-G., Li, X., Ji, S. & Lee, J. ChEST: A novel model measuring both Chemical Exchange and Susceptibility Tensor from resonance frequency shift. in International Society of Magnetic Resonance in Medicine (ISMRM) 29th Annual Meeting 4 (2021).
18. Liu, C. Susceptibility tensor imaging. Magnetic Resonance in Medicine 63, 1471–1477 (2010).
19. Li, X. & Zijl, P. C. M. van. Mean magnetic susceptibility regularized susceptibility tensor imaging (MMSR-STI) for estimating orientations of white matter fibers in human brain. Magnetic Resonance in Medicine 72, 610–619 (2014).
20. Tournier, J.-D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Figures
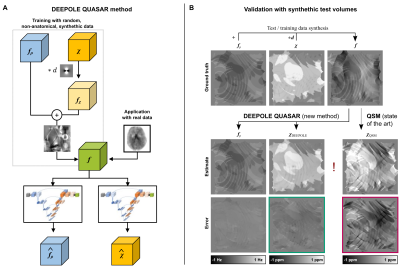
Fig. 1. (A) DEEPOLE QUASAR uses model-based training data synthesis for training two independent neural networks to map total frequency contrast f to the two source distributions χ and fρ, respectively. (B) The in silico validation shows that DEEPOLE QUASAR accurately disentangled and reconstructed the two sources (left; representative example). The QSM estimate of the magnetic susceptibility was much stronger affected by the fρ contributions (right), as illustrated by the error map for the QSM solution (red) in comparison with DEEPOLE QUASAR (green).
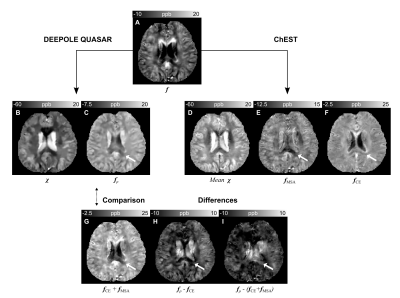
Fig. 2. Comparison between DEEPOLE QUASAR and ChEST. Depending on the region, fρ (C) shared similarities with fCE (F) and fMSA (E). While the WM-CSF contrast in fρ was similar to fCE, WM-GM and the highly anisotropic corpus callosum (arrow) required the addition of fCE and fMSA (G). Compared with ChEST’s mean magnetic susceptibility (D) from 12 orientations, DEEPOLE QUASAR’s estimate of the apparent isotropic susceptibility (B) under a single orientation is markedly lower in the white matter.
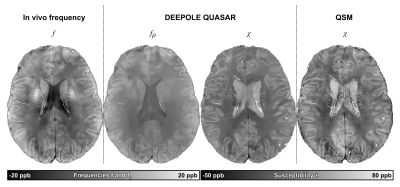
Fig. 3. DEEPOLE QUASAR and QSM solutions from a volunteer (f, 33y.). fρ shows non-susceptibility frequency to be higher in GM than in WM, and lowest in CSF. DEEPOLE QUASAR’s estimate of χ within the WM was more homogenous than the estimation from QSM (mean per-subject standard deviation in the WM across all 12 subjects: DEEPOLE QUASAR: 14.45±1.17 ppb, QSM: 22.06±2.23 ppb, p<0.001). [Data from this subject was previously presented at ISMRM.16]
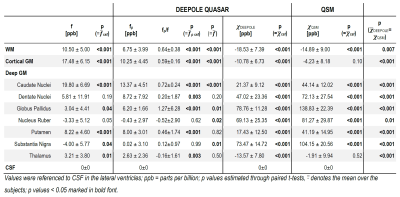
Table 1. Mean region-of-interest values of f, χDEEPOLE and fρ estimated with DEEPOLE QUASAR, and χQSM estimated with conventional QSM from volunteers (N=12, 3 T).