3306
Consistent White Matter Parcellation in Adolescent Brain Cognitive Development (ABCD): A ~10k Harmonized Diffusion MRI Study1Harvard Medical School, Boston, MA, United States
Synopsis
We present a large-scale harmonized dMRI study where we have performed successful white matter(WM) tractography parcellation across ~10k subjects from the ABCD study. We first assess the effects of data harmonization on WM parcellation, followed by an evaluation of WM parcellation using the entire harmonized dataset. We show that after harmonization, more anatomical tracts are identified and their FA values are closer to the harmonization reference data. We also demonstrate highly successful WM parcellation, where overall 99.9% of tracts(73 per subject) are identified. The parcellated WM tracts, as well as their diffusion measures, will be made available to the public.
INTRODUCTION
Diffusion MRI (dMRI) is the only non-invasive method that can map the living human brain’s connections1,2. Several large studies, such as the Adolescent Brain Cognitive Development (ABCD)3 Study, have acquired dMRI data from many thousands of subjects. However, it is a challenging task to analyze data collected from different scanners due to large inter-scanner (inter-site) differences for joint analysis of large-scale datasets. A second challenge for large-scale data analysis is the need for automated extraction of white matter (WM) connections across different populations in health and disease.We present a large-scale harmonized diffusion imaging study where we have performed successful WM tractography parcellation across ~10k subjects from the ABCD study acquired at 21 different sites. We first assess the effects of dMRI harmonization on WM parcellation, followed by an evaluation of the WM parcellation results using the harmonized data across the entire dataset. The parcellated WM tracts, as well as their tract measures derived from dMRI, will be made available via the NIH NDA repository. We believe that this will provide useful data sources for a large-scale data-intensive analysis of WM connections to study neurodevelopment.
METHODS
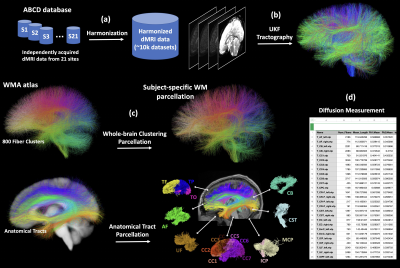
Figure-1 gives an overview of the major computational steps, including 1) dMRI harmonization, 2) whole-brain tractography, 3) white matter parcellation, and 4) diffusion measurement extraction.dMRI harmonization is performed using our novel machine learning algorithms that reconcile raw dMRI signals across disparate sites and acquisition parameters, while preserving inter-subject biological variability4. To this end, a reference site was selected and raw dMRI data from the remaining 20 sites were mapped to this reference.
Tractography is performed using our advanced multi-tensor unscented Kalman filter (UKF) fiber tracking algorithm5–7. UKF tractography accounts for crossing fibers, and it sensitively and consistently traces fibers across various populations8 and has enabled many studies of tract microstructure9–11.
WM parcellation is performed using our WhiteMatterAnalysis (WMA)12 pipeline13,14, in conjunction with an anatomical WM atlas8, for automated fiber clustering and extraction of anatomical tracts. This pipeline produces a fine-scale whole-brain tractography parcellation into 800 fiber clusters and a coarse-scale anatomical tract parcellation including 57 major deep WM tracts and 16 categories of superficial fiber clusters organized according to the brain lobes they connect (see Figure-2 for a list of anatomical tracts).
Multiple diffusion measurements are extracted from each parcellated cluster and anatomical tract using SlicerDMRI15,16. These include widely used measurements such as fractional anisotropy (FA) and the number of fibers (NoF). The tensors associated with each fiber point are also provided for computation of additional measures of interest.
Computation:
For processing this large-scale dataset, we use the Amazon EC2 platform. r5d.large EC instances (16GiB of memory, 2 vCPUs) are used, where tractography and WM parcellation took ~4.5 and 1.6 hours, respectively, per subject.
Experiments:
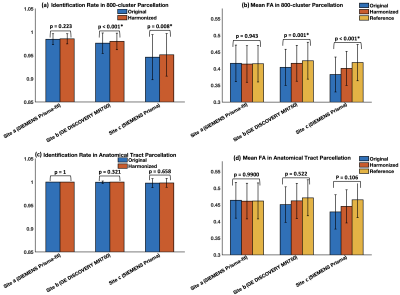
We first assess the effects of dMRI harmonization on WM parcellation, in three example sites acquired with different scanners. We perform analysis at both cluster- and tract-levels. For each cluster (or tract) per site, we measure its identification rate8,17 before and after harmonization. Here, a cluster (or a tract) is considered to be successfully identified if there are at least 10 fibers8,17,18, and its identification rate is defined as the percentage of successfully identified clusters (or tracts) across all analyzed subjects. In addition, for each cluster (or tract), we also compute the mean FA across all subjects per site before and after harmonization, in comparison to the reference data (to which all datasets were harmonized).
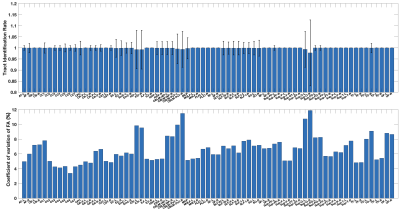
We then assess the WM parcellation performance across all ~10k harmonized dMRI datasets. For each cluster (or tract), we measure the mean identification rate and the coefficient of variation (CoV) of FA across all harmonized datasets. A lower CoV represents a low variability and thus reflects a high consistency of the parcellated clusters (or tracts) across subjects19,20.
Results
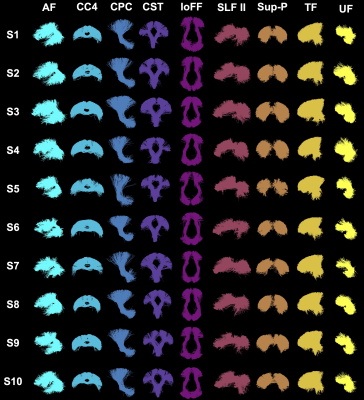
Figure-3 shows the effects of data harmonization on WM parcellation. After harmonization, the identification rates in both cluster- and tract-level parcellations (Figure-3(a,c)) increase across all three example sites, except for site-a where both original and harmonized data have 100% tract identification rate. The mean cluster and tract FA values (Figure-3(b,d)) are also closer to the corresponding reference data.Overall, across all ~10k harmonized dMRI data from 21 sites in the ABCD study, we obtain a high identification rate in both tract- and cluster-level parcellation (99.9% and 97.5%, respectively). We also obtain a low CoV of FA in both levels, 6.59% and 10.3%, respectively, which are low values indicative of consistent parcellation (CoV below 30% generally suggests a good data distribution21). Figure-4 gives the identification rate and CoV of FA for each of the 73 anatomical tracts. Figure-5 gives a visualization of example tracts from 10 randomly selected subjects from 10 different sites.
DISCUSSION & CONCLUSION
We presented a study of successful WM parcellation in a large-scale harmonized dMRI dataset across ~10k subjects from the ABCD study. We showed that the dMRI harmonization improves WM parcellation to obtain a higher identification rate and a closer FA to the harmonization reference data. We demonstrated successful WM parcellation across all harmonized datasets, where over 99.9% of the anatomical tracts were successfully identified. Visualization of tracts showed visually highly similar results across different subjects.Acknowledgements
R01MH125860, R01MH119222, R01MH074794, and P41EB015902References
1. Basser, P. J., Pajevic, S., Pierpaoli, C., Duda, J. & Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 44, 625–632 (2000).
2. Zhang, F. et al. Quantitative mapping of the brain’s structural connectivity using diffusion MRI tractography: a review. arXiv [q-bio.QM] (2021).
3. Casey, B. J. et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 32, 43–54 (2018).
4. Cetin Karayumak, S. et al. Retrospective harmonization of multi-site diffusion MRI data acquired with different acquisition parameters. Neuroimage 184, 180–200 (2019).
5. Malcolm, J. G., Shenton, M. E. & Rathi, Y. Filtered multitensor tractography. IEEE Trans. Med. Imaging 29, 1664–1675 (2010).
6. Reddy, C. P. & Rathi, Y. Joint Multi-Fiber NODDI Parameter Estimation and Tractography Using the Unscented Information Filter. Front. Neurosci. 10, 166 (2016).
7. ukftractography, https://github.com/pnlbwh/ukftractography
8. Zhang, F. et al. An anatomically curated fiber clustering white matter atlas for consistent white matter tract parcellation across the lifespan. Neuroimage 179, 429–447 (2018).
9. Olszewski, A. K. et al. The social brain network in 22q11.2 deletion syndrome: a diffusion tensor imaging study. Behav. Brain Funct. 13, 4 (2017).
10. Zhang, F. et al. Whole brain white matter connectivity analysis using machine learning: An application to autism. Neuroimage 172, 826–837 (2018).
11. Hamoda, H. M. et al. Abnormalities in thalamo-cortical connections in patients with first-episode schizophrenia: a two-tensor tractography study. Brain Imaging Behav. 13, 472–481 (2019).
12. whitematteranalysis: https://github.com/SlicerDMRI/whitematteranalysis
13. O’Donnell, L. J. & Westin, C.-F. Automatic tractography segmentation using a high-dimensional white matter atlas. IEEE Trans. Med. Imaging 26, 1562–1575 (2007).
14. O’Donnell, L. J., Wells, W. M., III, Golby, A. J. & Westin, C.-F. Unbiased Groupwise Registration of White Matter Tractography. in Medical Image Computing and Computer-Assisted Intervention, 123–130 (2012).
15. Norton, I. et al. SlicerDMRI: Open Source Diffusion MRI Software for Brain Cancer Research. Cancer Res. 77, e101–e103 (2017).
16. Zhang, F. et al. SlicerDMRI: Diffusion MRI and Tractography Research Software for Brain Cancer Surgery Planning and Visualization. JCO Clin Cancer Inform (2020).
17. Zhang, F. et al. Deep white matter analysis (DeepWMA): Fast and consistent tractography segmentation. Med. Image Anal. 65, 101761 (2020).
18. Zhang, F. et al. Creation of a novel trigeminal tractography atlas for automated trigeminal nerve identification. Neuroimage 220, 117063 (2020).
19. Zhang, F. et al. Comparison between two white matter segmentation strategies: An investigation into white matter segmentation consistency. in 2017 IEEE 14th International Symposium on Biomedical Imaging, 796–799 (2017).
20. Roberts, J. A., Perry, A., Roberts, G., Mitchell, P. B. & Breakspear, M. Consistency-based thresholding of the human connectome. Neuroimage 145, 118–129 (2017).
21. Brown, C. E. Coefficient of Variation. in Applied Multivariate Statistics in Geohydrology and Related Sciences (ed. Brown, C. E.) 155–157 (Springer Berlin Heidelberg, 1998).
Figures