3304
A deep learning diffusion MRI registration method using joint whole-brain and tract-specific information1Harvard Medical School, Boston, MA, United States
Synopsis
We present a novel deep learning method, DDMReg, for accurate dMRI registration. In dMRI registration, the goal is to align brain anatomical structures while ensuring local fiber orientations consistency with the underlying white matter anatomy. DDMReg is an unsupervised method for deformable dMRI registration, without the need of non-rigidly pre-registered images and the corresponding deformation field as ground truth. We propose a novel registration architecture that leverages not only whole-brain information but also tract-specific fiber orientation information. We perform comparisons with four state-of-the-art methods on several independently acquired datasets. Experimental results show that DDMReg obtains highly improved registration performance.
INTRODUCTION
Diffusion MRI (dMRI) is an advanced imaging technique that can measure the random diffusion of water molecules in the brain1. Registration of dMRI data is a crucial step in applications such as group analyses of dMRI data2,3 and construction of brain atlases4–6. Registration of dMRI data is a challenging task because it requires alignment of not only the anatomical structures of the entire brain but also local fiber orientations to ensure the consistency of the underlying white matter (WM) anatomy after image transformations7–10.We present a deep learning method for registration of dMRI data, namely Deep Diffusion MRI Registration (DDMReg). We propose a novel registration architecture that leverages not only whole brain information but also tract-specific fiber orientation information. DDMReg enables deformable registration to account for local nonlinear differences of the brains from different individuals, while the training process requires only rigidly aligned images but does not require nonlinearly pre-registered training data. We trained our model using high-quality Human Connectome Project (HCP)11 datasets and demonstrated a successful application to data acquired from different populations and different imaging protocols.
METHODS
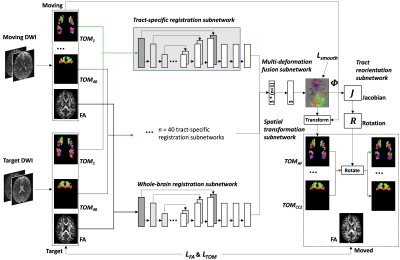
Figure-1 gives the method overview. From the dMRI data, we compute two types of input data, including: 1) FA, which is useful for aligning anatomical structures from the whole brain, and 2) 40 tract orientation maps (TOMs)12, which are useful for aligning fibers of specific anatomical WM tracts. For each type of input data, a U-net-based registration subnetwork (as used in13) is trained to compute a deformation field specific to the input data type. A multi-deformation fusion subnetwork is used to combine all deformation fields to estimate the final deformation field. One benefit of the multi-deformation fusion subnetwork is that the different registration subnetworks constrain each other, while cooperating to optimize the entire network. Following that, a spatial transformation subnetwork14 and a tract reorientation subnetwork are used to warp the moving data into the target space. Given the large number of registration subnetworks (a total of 41) that are difficult to fit in the GPU at once, we pretrain model weights of the tract-specific registration subnetworks, and we use them as backbones in our overall network.Experiments:
We demonstrate our method using dMRI datasets from multiple independently acquired populations, including: 1) 100 young healthy adults (29.1±3.7 years) in HCP11, 2) 30 teenagers (10.2±0.6 years) in Adolescent Brain Cognitive Development (ABCD)15, and 3) 30 elderly adults (63.1±5.6 years) in Parkinson’s Progression Markers Initiative (PPMI)16. These datasets were scanned with different imaging protocols.
The high-quality HCP datasets are used to train the registration model (n=50), as well as for validation (n=20) and testing (n=30). The ABCD and PPMI datasets, where dMRI data were obtained with a clinical acquisition protocol from studies geared towards clinical applications, are used to test how the trained model generalizes to data from different populations and different acquisition protocols.
We compare DDMReg to four state-of-the-art methods, including SyN17, DTI-TK8, MRRegister18, and VoxelMorph13. The experimental comparison is performed using the 30 HCP, 30 ABCD and 30 PPMI testing datasets.
We evaluate the registration performance using three metrics, including 1) tract Dice that quantifies volumetric overlap of the 40 WM tracts, 2) tissue Dice that quantifies volume overlap of the brain tissue segmentations (i.e., GM, WM, and CSF), and 3) tract distance that assesses fiber spatial alignment of the 40 WM tracts.
RESULTS
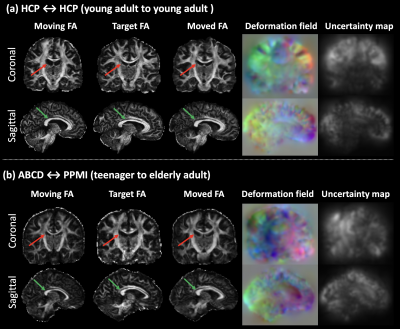
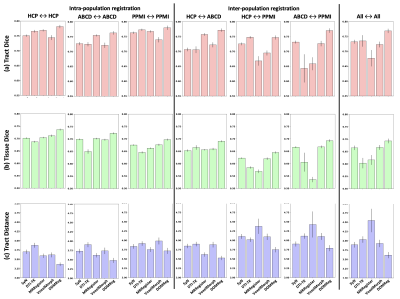
Figure-2 gives a visualization result of the proposed DDMReg method, including FA, deformation field, and registration uncertainty map (computed from the 40 tract-specific deformation fields). The deformation field is visually smooth, showing a regular, smooth displacement. The uncertainty map shows no tract-specific effects (e.g., no particular tract regions showing high uncertainty), even though our tract-specific registration subnetworks register based on individual tracts.Figure-3 shows that DDMReg obtains overall the best results (the highest tract Dice, the highest tissue Dice, and the lowest tract distance) across all compared methods in the “all-to-all” evaluation. In the evaluation of the intra- and inter-population subject pairs, DDMReg also achieves the best results in each of the six categories except for that DTI-TK obtains a slightly higher tract Dice than DDMReg in registration between HCP and PPMI (0.7475 versus 0.7469).
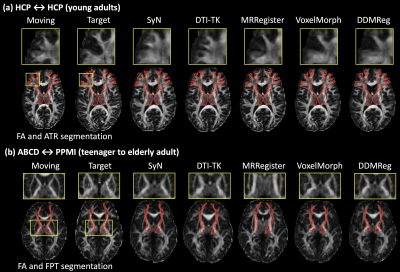
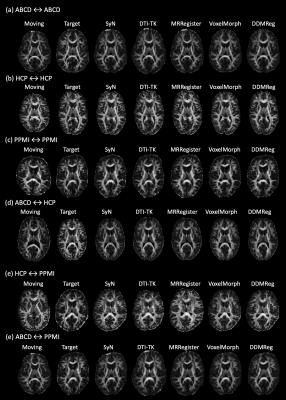
Figure-4 gives a visualization of registration results for each compared method, and Figure-5 gives visualization of more example image pairs from different datasets. In general, all methods generate visually good results to align the two brains, while we can observe improvements in local regions using DDMReg, in particular when registering data from populations with different ages and/or scanned with different protocols (e.g., Figure-4(b)).
CONCLUSION & DISCUSSION
We present a novel deep learning method to enable accurate dMRI registration. We design a novel network architecture for dMRI registration, which enables the ensemble of multiple registration subnetworks trained on different types of input data to form one unified prediction. Our method benefits from the unique tract-specific fiber orientation information encoded in TOMs. We demonstrate advanced dMRI registration performance compared to several state-of-the-art methods. Importantly, we show successful generalization of DDMReg to data from different populations and different scanners.Acknowledgements
P41 EB015902, P41 EB015898, R01 MH074794, R01 MH125860, P41 EB028741, and R01 MH119222References
1. Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267 (1994).2. Cetin-Karayumak, S. et al. White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol. Psychiatry 25, 3208–3219 (2020).
3. Moulton, E. et al. Comparison of spatial normalization strategies of diffusion MRI data for studying motor outcome in subacute-chronic and acute stroke. Neuroimage 183, 186–199 (2018).
4. Varentsova, A., Zhang, S. & Arfanakis, K. Development of a high angular resolution diffusion imaging human brain template. Neuroimage 91, 177–186 (2014).
5. Zhang, F. et al. An anatomically curated fiber clustering white matter atlas for consistent white matter tract parcellation across the lifespan. Neuroimage 179, 429–447 (2018).
6. Yeh, F.-C. et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. Neuroimage 178, 57–68 (2018).
7. Alexander, D. C., Pierpaoli, C., Basser, P. J. & Gee, J. C. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans. Med. Imaging 20, 1131–1139 (2001).
8. Zhang, H., Yushkevich, P. A., Alexander, D. C. & Gee, J. C. Deformable registration of diffusion tensor MR images with explicit orientation optimization. Med. Image Anal. 10, 764–785 (2006).
9. Duarte-Carvajalino, J. M., Sapiro, G., Harel, N. & Lenglet, C. A Framework for Linear and Non-Linear Registration of Diffusion-Weighted MRIs Using Angular Interpolation. Front. Neurosci. 7, 41 (2013).
10. Zhang, P., Niethammer, M., Shen, D. & Yap, P.-T. Large deformation diffeomorphic registration of diffusion-weighted imaging data. Med. Image Anal. 18, 1290–1298 (2014).
11. Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013).
12. Wasserthal, J., Neher, P., Hirjak, D. & Maier-Hein, K. H. Combined tract segmentation and orientation mapping for bundle-specific tractography. arXiv [cs.CV] (2019).
13. Balakrishnan, G., Zhao, A., Sabuncu, M. R., Guttag, J. & Dalca, A. V. VoxelMorph: A Learning Framework for Deformable Medical Image Registration. IEEE Trans. Med. Imaging (2019) doi:10.1109/TMI.2019.2897538.
14. Jaderberg, M., Simonyan, K., Zisserman, A. & Kavukcuoglu, K. Spatial Transformer Networks. arXiv [cs.CV] (2015).
15. Volkow, N. D. et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 32, 4–7 (2018).
16. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog. Neurobiol. 95, 629–635 (2011).
17. Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
18. Raffelt, D. et al. Symmetric diffeomorphic registration of fibre orientation distributions. Neuroimage 56, 1171–1180 (2011).
Figures