3302
An efficient deep learning framework for consistent superficial white matter tractography parcellation
Tengfei Xue1,2, Fan Zhang1, Chaoyi Zhang2, Yuqian Chen1,2, Yang Song3, Nikos Makris1, Yogesh Rathi1, Weidong Cai2, and Lauren Jean O’Donnell1
1Harvard Medical School, Boston, MA, United States, 2The University of Sydney, Sydney, Australia, 3University of New South Wales, Sydney, Australia
1Harvard Medical School, Boston, MA, United States, 2The University of Sydney, Sydney, Australia, 3University of New South Wales, Sydney, Australia
Synopsis
We propose a deep-learning-based framework, Superficial White Matter Analysis (SupWMA), which performs an efficient and consistent parcellation of 198 SWM clusters from whole-brain tractography. A point-cloud-based network is developed for our SWM parcellation task, and supervised contrastive learning enables more discriminative representations between plausible streamlines and outliers. SupWMA obtains highly consistent and accurate SWM parcellation results on a large tractography dataset with ground truth labels and on three independently acquired testing datasets from individuals across ages and health conditions.
Introduction
Diffusion magnetic resonance imaging (dMRI)1 allows the analysis of brain white matter connections via a computational process called tractography2. White matter parcellation classifies tractography streamlines into clusters or anatomically meaningful tracts to enable quantification and visualization. Most parcellation methods focus on the deep white matter (DWM) that traverses the depth of the white matter and connects remote cortical areas3–7, while few methods can parcellate the superficial white matter (SWM) that covers short-range association connections (u-fibers) connecting adjacent gyri8–11 due to its complexity. SWM tractography parcellation methods currently employ either ROI-based selection or streamline clustering. However, ROI-based methods8,12 highly depend on ROI parcellation schemes, and there are still challenges for clustering methods in achieving consistent parcellation and reducing runtime9,11,13. Recently, deep-learning-based methods5–7,14,15 have achieved promising results in tractography parcellation, but none have been developed for SWM parcellation.We propose a novel deep learning framework, Superficial White Matter Analysis (SupWMA), for SWM parcellation from whole-brain tractography. SupWMA is designed based on a point-cloud-based network16 with supervised contrastive learning17 to classify streamlines into SWM clusters and remove outliers. Our point-cloud-based network can have the same global representation for streamlines with equivalent forward and reverse point orders (e.g., from cortex to subcortical structures or vice versa). Supervised contrastive learning enables more discriminative representations between plausible streamlines and outliers. SupWMA obtains fast and consistent results on testing datasets across populations and dMRI acquisitions.
Methods
Our network (Figure 1 (a)) is based on PointNet16 and it can be divided into two parts: the encoder Enc(·) that extracts the global feature for each streamline and the classifier Cla(·) that predicts the streamline label. We remove transformation nets from PointNet to preserve spatial position of streamlines and improve the computational speed. For our Enc(·), the input is the Right-Anterior-Superior (RAS) coordinates of streamline points. A shared MLP of three layers individually encodes each point on a streamline. Then, a symmetric function (max-pooling16) aggregates the encoded features into a global shape feature (g) of the streamline. Because g is invariant to the order of points along a streamline, streamlines with equivalent forward and reverse point orderings will have the same global shape feature. Finally, Cla(·), consisting of three FC layers, is used for streamline class prediction.Supervised contrastive learning (SCL)17 is an extension of self-supervised contrastive learning19. In our task, streamlines in SWM outliers can be geometrically similar to those in SWM clusters, SCL assists Enc(·) in extracting more discriminative features between plausible streamlines and outliers. In SCL training (Figure 1 (b)), a projector head Proj(·)17,19 with two additional FC layers is added on Enc(·). Proj(·) may retain more instance-specific streamline information in the global feature, benefiting downstream tasks19. The supervised contrastive loss is as follows:
$$\mathcal{L}^{sup}_{out}=\sum_{i \in I}\mathcal{L}^{sup}_{out,i}=\sum_{i \in I} \frac{-1}{|P(i)|} \sum_{p \in P(i)} \log \frac{\exp ( z_{i} \cdot z_{p} / \tau )}{\sum_{a \in A(i)} \exp ( z_{i} \cdot z_{a} / \tau )}$$
where I is the streamline set in a training batch (i ∈ I ≡ {1, …, M}); P(i) is the streamline set that has the same class label as streamline i (p ∈ P(i)); A(i) is the set of all other streamlines in I except for streamline i (a ∈ A(i) ≡ I \ {i}); zi, zp and za are contrastive features from Proj(·) for streamlines i, p and a; τ (temperature) is a pre-defined hyperparameter.
The training procedure has two phases17,19 (Figure 1 (b)). In the SCL phase, Enc(·) and Proj(·) are trained with supervised contrastive loss. In the downstream learning phase, Enc(·)’s parameters are frozen17, and Proj(·) is unused. Cla(·) is trained with cross-entropy loss for streamline classification. A high-quality large-scale dataset with 1 million labeled streamlines (198 SWM clusters and one “non-SWM cluster") was used for model training and validation. This dataset was derived from an anatomically curated white matter tractography atlas11 (Figure 2).
Figure 1 (c) demonstrates parcellation of unlabeled tractography data (i.e., the testing data). We tested SupWMA using publicly available data (150 subjects) from three independently acquired datasets20–22 (Figure 2).
Results
Compared to two SOTA methods (Figure 3), SupWMA achieves the highest mean accuracy and macro F1-score with the lowest standard deviation. Also, FLOPs of SupWMA are much lower than other SOTA methods. Figure 4 gives the comparison results using the cluster identification rate (CIR)6,11 that measures the success of white matter cluster identification. Our method performs the best on all three testing datasets compared to three SOTA methods. Figure 5 shows visualization results of example clusters for each testing dataset and each method. All methods perform relatively well on clusters shown in magenta and cyan (except DeepWMA on HCP). However, SupWMA identifies more anatomically reasonable streamlines (yellow clusters) than other methods on the HCP and ABCD datasets.Discussion
The quantitative results demonstrate that our proposed method has good performance on datasets with ground truth (atlas) and can generalize well to obtain highly consistent SWM parcellation results for subjects across populations. The cluster visualization results show visually successful identification of SWM clusters across datasets.Conclusion
We proposed SupWMA, a novel deep learning framework for SWM parcellation, with successful application on datasets across ages and health conditions. SupWMA enables consistent and efficient SWM parcellation.Acknowledgements
R01MH125860, R01MH119222, R01MH074794, and P41EB015902References
1. Basser, P. J., Mattiello, J. & LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66, 259–267 (1994).2. Basser, P. J., Pajevic, S., Pierpaoli, C., Duda, J. & Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine vol. 44 625–632 (2000).
3. Yendiki, A. et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinform. 5, 23 (2011).
4. Garyfallidis, E. et al. Recognition of white matter bundles using local and global streamline-based registration and clustering. Neuroimage 170, 283–295 (2018).
5. Wasserthal, J., Neher, P. & Maier-Hein, K. H. TractSeg - Fast and accurate white matter tract segmentation. Neuroimage 183, 239–253 (2018).
6. Zhang, F. et al. Deep white matter analysis (DeepWMA): Fast and consistent tractography segmentation. Med. Image Anal. 65, 101761 (2020).
7. Chen, Y. et al. Deep Fiber Clustering: Anatomically Informed Unsupervised Deep Learning for Fast and Effective White Matter Parcellation. in Medical Image Computing and Computer Assisted Intervention – MICCAI 2021 497–507.
8. Oishi, K. et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43, 447–457 (2008).
9. Román, C. et al. Clustering of Whole-Brain White Matter Short Association Bundles Using HARDI Data. Front. Neuroinform. 11, 73 (2017).
10. Guevara, M., Guevara, P., Román, C. & Mangin, J.-F. Superficial white matter: A review on the dMRI analysis methods and applications. Neuroimage 212, 116673 (2020).
11. Zhang, F. et al. An anatomically curated fiber clustering white matter atlas for consistent white matter tract parcellation across the lifespan. Neuroimage 179, 429–447 (2018).
12. Ouyang, M. et al. Global and regional cortical connectivity maturation index (CCMI) of developmental human brain with quantification of short-range association tracts. Proc. SPIE Int. Soc. Opt. Eng. 9788, (2016).
13. Guevara, P. et al. Automatic fiber bundle segmentation in massive tractography datasets using a multi-subject bundle atlas. Neuroimage 61, 1083–1099 (2012).
14. Astolfi, P. et al. A Stem-Based Dissection of Inferior Fronto-Occipital Fasciculus with A Deep Learning Model. in 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI) 267–270 (2020).
15. Xu, H. et al. Objective Detection of Eloquent Axonal Pathways to Minimize Postoperative Deficits in Pediatric Epilepsy Surgery using Diffusion Tractography and Convolutional Neural Networks. IEEE Trans. Med. Imaging 38(8), 1910-1922 (2019).
16. Charles, R. Q., Su, H., Kaichun, M. & Guibas, L. J. PointNet: Deep Learning on Point Sets for 3D Classification and Segmentation. in 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR) 77–85 (2017).
17. Khosla, Prannay, et al. Supervised Contrastive Learning. Advances in Neural Information Processing Systems (NeurIPS) 33 (2020).
18. O’Donnell, L. J. & Westin, C.-F. Automatic tractography segmentation using a high-dimensional white matter atlas. IEEE Trans. Med. Imaging 26, 1562–1575 (2007).
19. Chen, T., Kornblith, S., Norouzi, M. & Hinton, G. A Simple Framework for Contrastive Learning of Visual Representations. in Proceedings of the 37th International Conference on Machine Learning. vol. 119 1597–1607 (PMLR, 2020).
20. Van Essen, D. C. et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79 (2013).
21. Volkow, N. D. et al. The conception of the ABCD study: From substance use to a broad NIH collaboration. Dev. Cogn. Neurosci. 32, 4–7 (2018).
22. Parkinson Progression Marker Initiative. The Parkinson Progression Marker Initiative (PPMI). Prog. Neurobiol. 95, 629–635 (2011).
23. Liu, F. et al. DeepBundle: Fiber Bundle Parcellation with Graph Convolution Neural Networks. in Graph Learning in Medical Imaging 88–95 (2019).
24. Ngattai Lam, P. D. et al. TRAFIC: Fiber Tract Classification Using Deep Learning. Proc. SPIE Int. Soc. Opt. Eng. 10574, (2018).
Figures
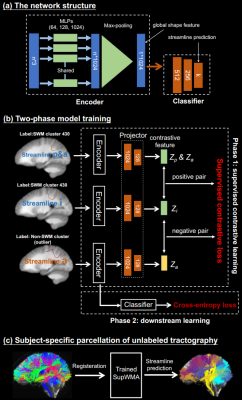
Figure 1. Method overview: (a) presents the structure of encoder and decoder. Dimensions of network layers and features are indicated. n (the number of points) is 156,11,18, and k (the number of classes) is 199. (b) gives our two-phase training procedure. Variables for supervised contrastive learning are consistent with the equation. (c) is the process of unlabeled tractography parcellation. Trained model takes the transformed tractography (in atlas space) and predicts class labels for each streamline.
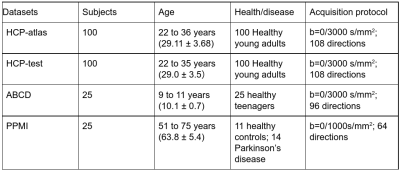
Figure 2. Demographics of the evaluation datasets. HCP-atlas (the training dataset)11 is derived from 100 subjects in the Human Connectome Project (HCP)20. HCP-test (another 100 subjects from HCP), Adolescent Brain Cognitive Development (ABCD)21 and Parkinson’s Progression Markers Initiative (PPMI)22 datasets are used for evaluation. These evaluation datasets (testing) are across ages, health conditions and acquisition protocols.
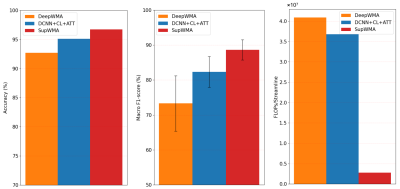
Figure 3. Results on datasets with ground truth are illustrated. 5-fold cross-validation is used for evaluation. We compare SupWMA with other two deep-learning-based state-of-the-art (SOTA) tractography parcellation methods: DCNN+CL+ATT15 and DeepWMA6. As widely used metrics6,15,23,24 for tractography parcellation, accuracy and macro F1-score are reported. Floating point operations (FLOPs) measure the number of required operations in model inference (efficiency)16.
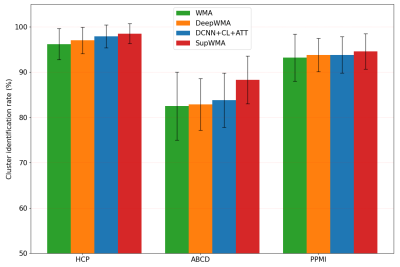
Figure 4. Cluster identification rate (CIR) across methods for three datasets are shown. In addition to DCNN+CL+ATT and DeepWMA, we include another SOTA method, WMA11,18. CIR indicates the percentage of detected clusters in a subject when ground truth labels are not available. Here, a cluster containing at least 20 streamlines6,11 is considered to be successfully detected.
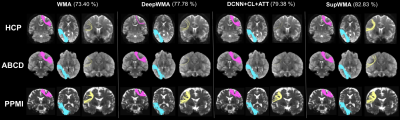
Figure 5. Visualization of example individual clusters is presented. For each testing dataset, the subject that has the lowest CIR is selected for visualization. The average CIR (across the three subjects) is displayed for each method.
DOI: https://doi.org/10.58530/2022/3302