3299
The effect of primed intravenous infusion of magnetic resonance contrast agent on diffusion tensor imaging in vivo1Advanced Imaging Research Center, UT Southwestern Medical Center, Dallas, TX, United States
Synopsis
The challenge of conducting diffusion tensor imaging in the presence of systemic contrast agent has been noted clinically. Here, we investigate primed infusion as a means of stabilizing contrast enhancement in the mouse brain during image acquisition. Our results show that with infusion-sustained contrast enhancement, fractional anisotropy (FA) was significantly elevated in whole brain and corpus callosum ROIs, but not in hippocampus ROI. Contrast enhancement significantly increased tractography metric of relative mean fiber-track length in corpus callosum versus hippocampus. Thus, contrast enhancement may have a preferential effect on anisotropic brain regions and could potentially be applied to amplify FA selectively.
INTRODUCTION
In clinical research, contrast agent administration may present a challenge to acquisition of diffusion tensor imaging (DTI) data, since rapid clearance of gadolinium-based agents in the blood would result in nonuniform concentration during image acquisition1,2. On the other hand, if contrast agent levels can be stabilized, it may be possible to take advantage of contrast enhancement for DTI.Previous DTI studies in the presence of contrast agent have only been done in the clinic with most subjects having brain tumors1–4 . Here, we address the problem of fluctuating gadolinium concentration through primed infusion, which has been shown to sustain contrast agent enhancement5. To our knowledge, in vivo DTI has not been studied under conditions of continuous infusion of contrast agent during imaging. The aim of the study herein, therefore, is to investigate diffusion tensor characteristics (here, fractional anisotropy) in the brains of normal mice under primed infusion of contrast agent.
METHODS
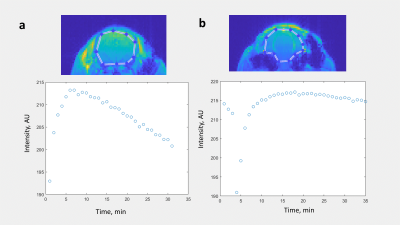
In primed infusion5, a bolus of contrast agent is injected intravenously, and after a delay of several minutes is followed by constant infusion of contrast agent. To optimize delivery parameters, T1-weighted scans were run with an initial bolus injection to observe contrast dynamics (Figure 1a). Based on this, we determined that infusion begin 5 min post bolus and continue for the duration of animal time on the scanner. After observing continuous constant intensity (>20 min, Figure 1b) in T1-weighted scans, the same contrast agent delivery parameters were used for diffusion tensor imaging (DTI) in an unused animal.To compare diffusion tensor data with and without contrast agent in each of n=4 male C57/BL6 mice, a DTI scan was performed with no contrast agent. Once the scan was complete, a 0.6 mmol bolus of Gadovist was injected, followed by initiation of infusion of 0.005 mmol/kg/min of the contrast agent 5 min post bolus delivery. A second DTI scan was delayed until 10 min after the start of infusion to allow the contrast agent to equilibrate. A DTI-EPI MRI sequence was used with 30 diffusion directions, TR/TE=4500/19.5 ms, NEX=3, scan time 32 min.
Data processing was conducted using Diffusion ToolKit and TrackVis (Martinos Center for Biomedical Imaging) to obtain diffusion tensor data and tractography. Datafiles were converted to Analyze format using DTIStudio (Johns Hopkins University, JHU). To compare data with and without contrast agent (in the same animal) pairwise t-tests were performed.
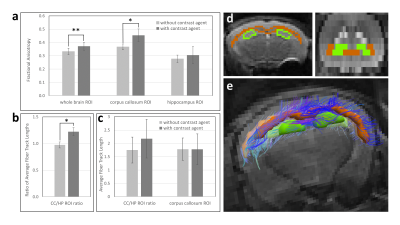
ROIs were drawn manually on B0 anatomical images in ROIEditor (JHU). The corpus callosum was selected as a known region of high fractional anisotropy (FA) while the hippocampus was chosen for its lower FA.
RESULTS
With primed infusion, contrast enhanced T1-shortening in the brain was kept constant (within ~2% intensity variation) for the duration of in vivo imaging of mice (Figure 1b). From DTI scans using the same contrast agent delivery parameters, mean FA values of whole brain ROIs was significantly elevated for the contrast agent scan (0.372 ±0.030 up from 0.333 ±0.025, p=0.00335, Figure 2a).Likewise, mean FA of ROIs defined within the corpus callosum was significantly elevated with contrast agent (0.454 ±0.047 up from 0.367 ±0.022, p=0.0327). However, in hippocampus ROIs, mean FA increase with contrast agent was not significant (0.305 ±0.064 up from 0.277 ±0.028, p=0.502, Figure 2a).
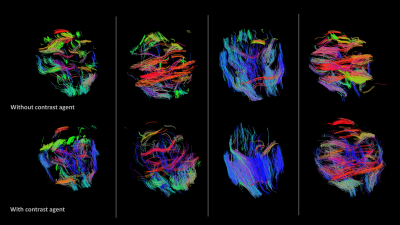
Tractography of brain white matter was also observed to be affected by contrast agent delivered in vivo during DTI scanning. While tractography metrics calculated for whole brain were not significantly different with contrast agent, qualitative differences are observable (Figure 3). For ROIs mentioned above, the ratio of mean fiber track length in the corpus callosum ROI to that in the hippocampal ROI increased with contrast agent (1.22 ±0.078 up from 0.97 ±0.056, p=0.0179, Figure 2b). While tractography metrics including track length, number of tracks and total track volume within each ROI did increase with gadolinium, they were not statistically significant (Figure 2c).
B0 maps were quantified for SNR changes with contrast agent and were found insignificant (p>0.3).
DISCUSSION
Based on these results, sustained contrast enhancement elevates brain FA overall, but may differ in effect by brain region. Based on ROI selection, contrast agent may have the effect of amplifying FA in anisotropic brain regions such as the corpus callosum, but have less effect on regions that are not anisotropic like the hippocampus. Results also showed that contrast agent has more effect on tractography of the corpus callosum than the hippocampus, consistent with FA results. It is noteworthy that the changes in FA occurred with no SNR increase.While gadolinium-based contrast agents are unable to cross the blood-brain barrier, their effect may result from extension of changes in the magnetic field beyond capillaries to white matter fiber bundles in proximity to the vessels. To further understand the effects of constant infusion, it may be useful to compare these results to DTI acquired with bolus contrast agent absent infusion.
CONCLUSIONS
Primed infusion can potentially solve the problem of fluctuating contrast agent levels when DTI can only be acquired in the presence of contrast agent as might be the case in a clinical setting. Beyond this, the data herein suggest that contrast agent could be used to selectively amplify FA in anisotropic regions of the brain.Acknowledgements
No acknowledgement found.References
1. Zolal, A. et al. The effect of a gadolinium-based contrast agent on diffusion tensor imaging. Eur. J. Radiol. 81, 1877–1882 (2012).
2. Bae, M. S., Jahng, G. H., Ryu, C. W. & Kim, E. J. A systematically designed study to investigate the effects of contrast medium on diffusion tensor MRI. J. Neuroradiol. 38, 214–222 (2011).
3. Bae, M. S. et al. Effect of intravenous gadolinium-DTPA on diffusion tensor MR imaging for the evaluation of brain tumors. Neuroradiology 51, 793–802 (2009).
4. Firat, A. K., Şanli, B., Karakaş, H. M. & Erdem, G. The effect of intravenous gadolinium-DTPA on diffusion-weighted imaging. Neuroradiology 48, 465–470 (2006).
5. Kalber, T. L. et al. Primed Infusion with Delayed Equilibrium of Gd.DTPA for Enhanced Imaging of Small Pulmonary Metastases. PLoS One 8, 1–8 (2013).
Figures