3298
Combining navigator-based prospective and data-driven retrospective motion correction for high resolution 7T multi-slice T2W MRI.1Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Amager and Hvidovre, Copenhagen, Denmark, 2Biomedical Image Technologies, ETSI Telecomunicación, Universidad Politécnica de Madrid and CIBER-BBN, Madrid, Spain, 3Philips Healthcare, Copenhagen, Denmark, 4Neurobiology Research Unit, Department of Neurology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark, 5Section for Magnetic Resonance, DTU Health Tech, Kgs Lyngby, Denmark, 6Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark, 7Lund University Bioimaging Center, Lund University, Lund, Sweden, 8Department of Radiology, Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark
Synopsis
High-resolution multi-slice T2W imaging at ultra-high field is highly sensitive to motion due to high tissue-CSF contrast and the k-space filling pattern. In this study we investigated the combination of 3D prospective and in-plane retrospective motion correction to improve image quality. We observed that a combination of navigator based prospective and data-driven retrospective correction is able to correct most severe motion artifacts during scanning, and remove smaller residual artifacts in reconstruction.
Introduction
High-resolution multi-slice T2W imaging, used in e.g. epilepsy, is highly sensitive to motion. This is due to both the high tissue-CSF contrast and the turbo-spin echo k-space filling, where every echo-train traverses the whole span of k-space (Figure 1). In typical high-resolution 7T-T2W, many short TSE trains are used to sample the image over 5-10 minutes. As a consequence, each displaced shot generates visible major artifacts (Figure 1c), putting stringent requirements on the accuracy of motion correction.Motion correction at ultra-high field has been shown (mainly for 3D sequences) with both prospective and retrospective approaches. Prospective correction can be performed with e.g. MRI navigators, which provides direct correction of motion without additional hardware (1). An open question is what level of accuracy can be reached for demanding applications, such as the high-resolution multi-slice T2W imaging used in this study.
Recently, innovations in retrospective motion correction have used purely data-driven correction where both the motion trace and image are jointly optimized in an iterative reconstruction (2-4). However, for multi-slice sequences this is challenging due to lack of through-plane information. It could however be valuable to correct residual in-plane motion after prospective correction is applied.
In this study, we investigated to what extent prospective motion correction corrects motion artifacts in a 7T multi-slice T2W sequence. Secondly, we investigated the additional value of retrospective motion correction.
Methods
The study was approved by the Scientific Ethical Committee of the Capital Region of Denmark (H-17013561) and all subjects signed consent forms prior to scanning.The scans were acquired at a 7T MRI system (Philips Healthcare, the Netherlands) with a 32/2 Rx/Tx quadrature coil (Nova Medical, US).
15 healthy volunteers were asked to perform either large stepwise head motion (by moving the nose in a triangle), jerk motion (by moving head to a different position and back) or secondary motion (by moving shoulders and legs). All three schemes followed a 1 minute still + 5 seconds movement paradigm throughout the scans. The different types of instructed motion resulted in different levels of loss in image quality. Examples of motion curves and image quality (without correction) are shown in figure 2.
A 9-echo TSE T2W multi-slice sequence with 32 slices, sampled coronally oblique to the hippocampi, TR/TE=3.5s/60ms, 0.5x0.5x1mm, 2 packages, 2 averages, SENSE=1.7, scan duration=5:50 min, prospectively motion corrected with 5 mm 3D fat-selective navigators (5) using the iMOCO framework (1) (SENSE 3x3, acqvol=500 ms), with reacquisition.
T2W scans were acquired with and without motion and with and without prospective correction (noMO-noCO, noMO-CO, MO-noCO and MO-CO).
These four scans subsequently underwent retrospective motion correction, resulting in 8 outcomes per subject. The additional retrospective motion correction was performed with aligned SENSE (2,3), which is an iterative solver that optimizes both the image and motion parameters for maximum consistency with the forward signal model, including signal encoding and receiver sensitivities. To reduce the parameter space, the model was limited to in-plane translations and rotation as it was assumed most through-plane motion would be corrected for by prospective correction.
$$[\hat{\theta},\hat{x}]=argmin_{\theta,x}\parallel s-E_\theta x\parallel_2$$
with s the acquired k-space signal (multicoil), x the reconstructed image and $$$E_\theta$$$ the forward model for motion $$$\theta$$$.
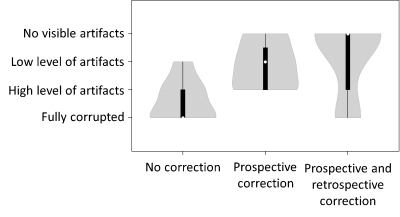
Image quality of the MO-noCO scans, the MO-CO scans, and the MO-CO scans with retrospective correction were assessed visually by a single reader and scored on a 4-level scale (fully corrupted, high level of motion artifacts, low level of motion artifacts, no visible motion artifacts).
Results
In cases with relatively low amounts of motion (mainly jerk and secondary motion), prospective motion correction alone restored most of the image quality (Figure 3). Additional retrospective motion correction further improved image quality in some cases.In the cases large stepwise motion, prospective motion correction reduced motion artifacts, but residual motion artifacts were observed in all 5 volunteers (Figure 4). In two of these, additional retrospective motion correction provided good data quality, in three residual artifacts became worse. This could be caused by persistent through-plane motion that was not corrected for by the prospective correction.
Overall, image quality scores were better using prospective correction over no correction. Additional retrospective correction led to further improvement over no correction, with equal or improved image quality compared to “prospective only” in most cases. (Figure 5)
Discussion
Prospective navigator-based motion correction can correct for small and modest motion in high-resolution T2W TSE at ultra-high field, but residual motion artifacts were still observed with large instructed motion. Additional data-driven retrospective correction improved image quality further in most cases. Reduction in image quality observed for three cases with large step-wise motion cases are ascribed to uncorrected through-plane motion during scanning. A retrospective correction including through-plane motion could potentially provide a solution, although inherently limited through-plane data is available due to slice gaps. For similar reasons, retrospective correction alone (without prospective correction) did not improve image quality in most cases, although we did observe improvements in cases with small motion.Conclusion
For high-resolution T2W imaging at ultra-high field, the combination of navigator-based prospective and data-driven retrospective correction showed to correct most motion artifacts during scanning, and remove small residual motion in reconstruction, respectively.Acknowledgements
The Danish national 7T scanner was donated by the John and Birthe Meyer Foundation and The Danish Agency for Science, Technology and Innovation (grant no. 0601-01370B).
References
1. Andersen M, Bjorkman-Burtscher IM, Marsman A, Petersen ET, Boer VO. Improvement in diagnostic quality of structural and angiographic MRI of the brain using motion correction with interleaved, volumetric navigators. PLoS One 2019;14(5):e0217145.2. Cordero-Grande L, Teixeira RP, Hughes E, Hutter J, Price A, Hajnal J. Sensitivity Encoding for Aligned Multishot Magnetic Resonance Reconstruction. IEEE Transactions on Computational Imaging 2016;2:1-1.
3. Cordero-Grande L, Hughes EJ, Hutter J, Price AN, Hajnal JV. Three-dimensional motion corrected sensitivity encoding reconstruction for multi-shot multi-slice MRI: Application to neonatal brain imaging. Magn Reson Med 2018;79(3):1365-1376.
4. Haskell MW, Cauley SF, Wald LL. TArgeted Motion Estimation and Reduction (TAMER): Data Consistency Based Motion Mitigation for MRI Using a Reduced Model Joint Optimization. IEEE Trans Med Imaging 2018;37(5):1253-1265.
5. Gallichan D, Marques JP, Gruetter R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Magn Reson Med 2016;75(3):1030-1039.
Figures
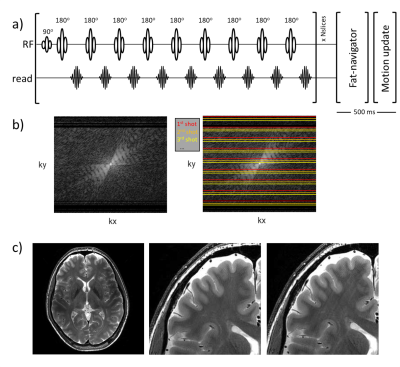
Figure 1. (a) 9-echo TSE sequence used for multi-slice T2W imaging at 7T. (b) Every shot is distributed along the ky axis so that every shot fills part of the center k-space. This makes that a small error in motion correction for each shot can produce significant image artifacts. A simulation of a single 1 mm motion in 1% of the data of a T2W TSE scan (1 out of 87 shots) (c), leads to visible motion artifacts (c, middle vs right). This illustrates the high motion sensitivity of the scan and the stringent constraint put on the motion correction accuracy.
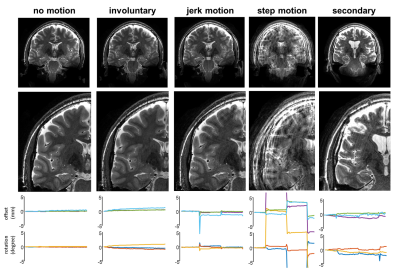
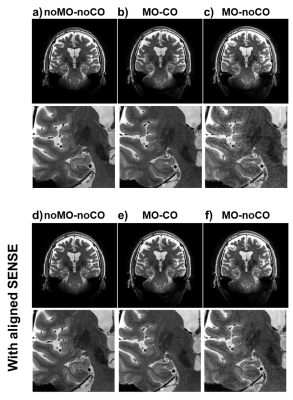
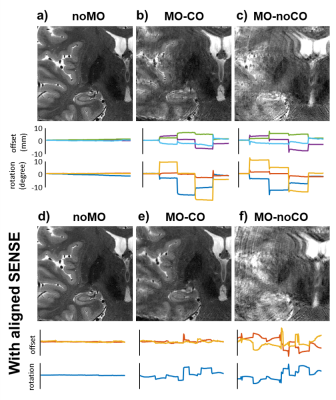
Figure 4. Case with relatively large motion and corresponding motion traces. In noMO-noCO (a) no artifacts are observed, whereas MO-noCO (c) shows severe motion artifacts. The MO-CO (b) showed improve quality, but image quality was not fully restored. Additional retrospective motion correction using aligned sense (c-f) showed no further improvement in image quality in noMO-noCO (a vs d) but showed improved image quality on top of the prospectively corrected scan (b vs e). The motion trace (e) confirms that in motion was persistent in the data even after prospective correction.