3295
Characterization of the Zona incerta and Fields of Forel using QSM at 9.4T1Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 2Biomedical Magnetic Resonance, University Hospital and Eberhard-Karl’s University, Tuebingen, Germany
Synopsis
The subthalamic structures, like the zona incerta (ZI), fields of forel (FF), subthalamic nucleus (STN), and their surrounding structures, are poorly characterized regarding anatomical imaging. Using quantitative susceptibility mapping (QSM) at ultra-high-field, iron, and myelin mapping may allow an advanced characterization. Therefore, QSM data were acquired at 9.4 Tesla in 21 subjects to characterize the para- and diamagnetic properties of the ZI, FF, and their adjacent structures. In contrast to the FF, we found a higher contribution of diamagnetic and paramagnetic sources, i.e., iron, myelin, and calcium, in the STN and ZI.
Introduction:
Introduction: Almost all the fiber tracts from the thalamus to the spinal cord intersect and cross via the zona incerta (ZI), fields of forel (FF), subthalamic nucleus (STN), medial lemniscus (ml), and red nucleus (RN) in the brainstem 1–4. Additionally, some fiber tracts pass between the thalamus and subcortex via ZI and FF. The area within the diencephalon, ventral to the thalamus, medial to the internal capsule, and lateral to the hypothalamus, is named subthalamus and encompasses grey matter nuclei and white matter structures5 and mainly consists of the zona incerta, the fields of forel, and the subthalamic nucleus.The ZI connects to the thalamus, basal ganglia, brainstem, spinal cord, and cortical structures and is implicated in gating sensory inputs, synchronizing cortical and subcortical brain rhythms, and limbic-motor integration, and controlling visceral activity and pain 1,2,4,6–14. Therefore, ZI also serves as a central target for deep brain stimulation (DBS) in Parkinson's disease 15.
The FF consist of three white matter areas known as "H fields." FF contains the fasciculus cerebello-thalamicus (fct), fasciculus lenticularis (fl), fasciculus thalamicus (ft), and the hf and hfield. The FF links the striatopallidal system with the thalamo-cortical network 16 and is a target for DBS in Tourette's syndrome 16. STN modulates basal ganglia output and connections with the cerebral cortex, thalamus, palladium, substantia nigra, brainstem, and striatum. The STN is also implicated in movement disorders 17 and is thus is also a DBS target in Parkinson's disease. Furthermore, as these subthalamic structures comprise densely myelinated connections, which intersect and encompass iron-rich nuclei, we tried to obtain a detailed map of the ZI, FF, STN nuclei using QSM data at 9.4 Tesla.
Material and Methods:
Subjects and MRI acquisition: 21 healthy volunteers without neurological disorders were screened through a qualified doctor's rigorous safety assessment and scanned at 9.4 T (Siemens Medical Solutions, Erlangen, Germany) using a 16-channel transmit/ 31-channel receive array 18. B1-mapping, anatomical MP2RAGE images 19 and mono-polar multi-echo 3D gradient echo (GRE) images with 5 echoes and echo times, TE=6-30ms in steps of 6ms; a repetition time TR=35 ms; and nominal FA=11° with a voxel size of 375x375x800 μm were acquired with an axial prescription.Preprocessing: QSM maps were preprocessed as described previously 20. The whole-slab phase-referencing was followed by VSHARP background removal and dipole inversion using STI-studio. After coregistration to the anatomical images, the QSM maps were normalized to MNI space and multiplied by 1000 to obtain standardized data in the ppb range.
Postprocessing: The anatomical analysis was performed using the MRI-based atlas of the subthalamic substructures 2. In the first step, the MNI spaced atlas was resliced to 400-micron native data resolution. Positive QSM values were assigned to paramagnetic maps, setting all other voxels to zero (>0) and vice versa; negative values (<0) were assigned to the diamagnetic maps. The next step, substructure-specific QSM means, was computed for the left and right hemispheres for the data extracted from the diamagnetic and paramagnetic maps. Violin and histogram plots were used for descriptive statistics in python to show mean values for all subjects in their respective comparisons (s. Fig. 2-4).
Results:
The standardized imaging data (in ppb) thresholded (at >0 & <0) for positive and negative values show diamagnetic sources, myelin, calcium, and paramagnetic sources separately as negative and positive values (s. Fig. 1a-c). The negative diamagnetic map shows a slightly homogeneous distribution of myelin-related QSM values within the subthalamus, in contrast, to the positive paramagnetic map (s. Fig 1a-c & 4). In addition, the violin plots of diamagnetic and paramagnetic sources depict subtle interindividual variabilities (s. Fig. 2 & 3). The average values of all subjects display that the distribution differs across ZI, FF, STN, and RN with slight laterality differences and reveals lower mean values for the FF and higher mean values for the ZI and STN. (s. Fig. 2 & 3). The latter most likely indicates a stronger contribution of diamagnetic and paramagnetic sources in the STN, RN, and ZI than in FF and ml (s. Fig. 4).Discussion:
The distribution of diamagnetic and paramagnetic sources is not uniform across the subthalamic structures (s. Fig 2-4). The ultra-high field gives good access to such subtle susceptibility effects. However, imaging subthalamus structures with ultra-high field remains challenging due to their small sizes and low signal-to-noise ratio. Furthermore, their delineation is affected by partial volume effects and individual variability.Conclusion:
This study reveals specific delineations of diamagnetic and paramagnetic sources in the ZI, FF, STN, ml, and RN. Interestingly, we observed an increase in susceptibility value in both sources for the STN and ZI compared to FF. We also found slight hemispheric differences. However, future work is required for a more comprehended relationship between QSM data and subthalamic structures. Furthermore, a larger sample of data is needed to examine the effect of age and gender to understand the subthalamus better.Acknowledgements
This work was supported by the DFG (Grant number GZ: GR 833/13-1). We thank Jonathan Lau for providing subthalamus masks.References
1. Barthó, P. et al. Cortical Control of Zona Incerta. J. Neurosci. 27, 1670–1681 (2007).
2. Lau, J. C. et al. Direct visualization and characterization of the human zona incerta and surrounding structures. Hum. Brain Mapp.41, 4500–4517 (2020).
3. Mitrofanis, J. & deFonseka, R. Organisation of connections between the zona incerta and the interposed nucleus. Anat. Embryol. (Berl.) 204, 153–159 (2001).
4. Watson, G. D. R., Smith, J. B. & Alloway, K. D. The Zona Incerta Regulates Communication between the Superior Colliculus and the Posteromedial Thalamus: Implications for Thalamic Interactions with the Dorsolateral Striatum. J. Neurosci. 35, 9463–9476 (2015).
5. Carpenter, M. B. Core Text of Neuroanatomy. (Williams & Wilkins, 1985).
6. Edwards, D. A. & Isaacs, S. Zona incerta lesions: effects on copulation, partner-preference and other socio-sexual behaviors. Behav. Brain Res. 44, 145–150 (1991).
7. Forel, A. Untersuchungen über die Haubenregion und ihre oberen Verknüpfungen im Gehirne des Menschen und einiger Säugethiere, mit Beiträgen zu den Methoden der Gehirnuntersuchung. Arch. Für Psychiatr. Nervenkrankh. 7, 393–495 (1877).
8. Lavallée, P. et al. Feedforward Inhibitory Control of Sensory Information in Higher-Order Thalamic Nuclei. J. Neurosci. 25, 7489–7498 (2005).
9. Ma, T. P. Saccade-related omnivectoral pause neurons in the primate zona incerta. NeuroReport 7, 2713–2716 (1996).
10. Masri, R. et al. Zona Incerta: A Role in Central Pain. J. Neurophysiol. 102, 181–191 (2009).
11. Mitrofanis, J. Some certainty for the "zone of uncertainty"? Exploring the function of the zona incerta. Neuroscience 130, 1–15 (2005).
12. Trageser, J. C. et al. State-Dependent Gating of Sensory Inputs by Zona Incerta. J. Neurophysiol. 96, 1456–1463 (2006).
13. Urbain, N. & Deschênes, M. Motor Cortex Gates Vibrissal Responses in a Thalamocortical Projection Pathway. Neuron 56, 714–725 (2007).
14. Zhang, X. & van den Pol, A. N. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science356, 853–859 (2017).
15. Plaha, P., Khan, S. & Gill, S. S. Bilateral stimulation of the caudal zona incerta nucleus for tremor control. J. Neurol. Neurosurg. Psychiatry 79, 504–513 (2008).
16. Neudorfer, C. et al. Deep Brain Stimulation of the H Fields of Forel Alleviates Tics in Tourette Syndrome. Front. Hum. Neurosci. 11, 308 (2017).
17. Hamani, C., Saint-Cyr, J. A., Fraser, J., Kaplitt, M. & Lozano, A. M. The subthalamic nucleus in the context of movement disorders. Brain J. Neurol. 127, 4–20 (2004).
18. Shajan, G. et al. A 16-channel dual-row transmit array in combination with a 31-element receive array for human brain imaging at 9.4 T. Magn. Reson. Med. 71, 870–879 (2014).
19. Hagberg, G. E. et al. Whole brain MP2RAGE-based mapping of the longitudinal relaxation time at 9.4T. NeuroImage 144, 203–216 (2017).
20. Hagberg, G., Eckstein, K., Cuna, E., & Scheffler, K. Towards robust QSM in cortical and sub-cortical regions of the human brain at 9.4T: influence of coil combination and masking strategies. in 2020 ISMRM & SMRT Virtual Conference & Exhibition. (2020).
Figures

Figure 1a: Zona incerta: QSM maps overlaid on MNI spaced T2w anatomical scans in MNI coordinates with a slice gap of 0.5. The figure depicts negative values for diamagnetic sources (myelin, calcium) and positive values for paramagnetic sources (iron) in parts per billion. The color code for paramagnetic sources is 'red-yellow, ' which higher values in yellow and lower values in red color. The color code for diamagnetic sources is inverted 'red-yellow, in which higher values in red and lower values in yellow color.

Figure 1b: Fields of Forel: The QSM maps overlaid on MNI spaced T2w anatomical scans with a slice gap of 0.5. The figure depicts negative values for diamagnetic sources (myelin, calcium) and positive values for paramagnetic sources (iron) in parts per billion. The depicted color code is similar to figure 1a.

Figure 1c: STN, RN, and ml: The QSM maps overlaid on MNI spaced T2w anatomical scans with a slice gap of 0.5 and depicted negative values for diamagnetic sources (myelin, calcium) and positive values for paramagnetic sources (iron) in parts per billion. The depicted color code is similar to figure 1a.
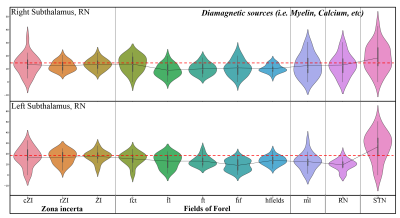
Figure 2: Violin plots of diamagnetic sources of the mean values of each substructure of the ZI, FF, STN. Note the inter-individual variability and laterality differences and the lower values (dotted red line) for the FF and higher values of the ZI and STN.
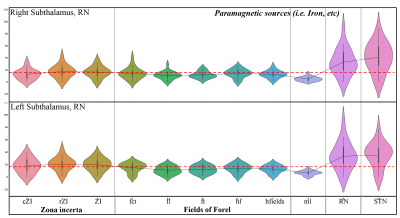
Figure 3: Violin plots of paramagnetic sources of the mean values of each substructure of the ZI, FF, STN. Note the inter-individual variability and laterality differences and the lower values (dotted red line) for FF, ml, and the higher values for ZI, RN, and STN.
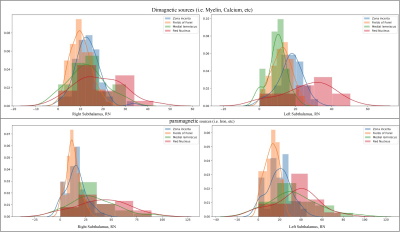
Figure 4: Histograms of the mean values for 21 subjects, considering diamagnetic (upper row) and paramagnetic (lower row) sources within ZI, FF, STN, ml, and RN. Each histogram shows values for the ZI are in blue, FF in orange, ml in green, and RN in red color. Note that the STN is not depicted as it has the highest values (intensity >50 fold higher). The STN shows the highest values, afterward RN, ZI, ml, FF in an overlapping descending order indicating the more substantial contribution of diamagnetic and paramagnetic sources, Iron, Myelin, and Calcium in ZI, FF, STN, ml, and RN.