3288
Hyperpolarized 15N-BBCP as a novel probe of H2O21Chemistry, Duke University, Durham, NC, United States, 2Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Philips Healthcare, Dallas, TX, United States, 4Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States
Synopsis
In this work, we have developed a novel de novo 15N-molecular probe, 15N-boronobenzyl-4-cyanopyridinium (15N-BBCP), for in vivo detection of H2O2. The long spin-lattice relaxation time (T1), high H2O2 sensitivity and selectivity, low cytotoxicity, and large chemical shift changes upon reaction suggest that the probe is suited for in vivo hyperpolarized 15N MRI.
Introduction
Reactive oxygen species (ROS), generated through oxidative metabolism, play important roles in regulating a wide range of biological and physiological processes. Balanced ROS homeostasis are requisites of cellular signaling pathways, yet accumulation of ROS leads to oxidative stress and has been a hallmark for cancer, inflammation, and neurodegenerative disorders.1,2 Among various ROS metabolites, hydrogen peroxide (H2O2) exists in highest concentration (10–7–10–8 M range) and high stability in normal physiology.3,4 The broad actions of H2O2 in pathophysiology highlight the need for a translatable imaging method with high spatiotemporal assessments for quantitative and dynamic detection of H2O2 in states of oxidative stress. Several molecular imaging agents for H2O2 sensing have been disclosed, including hyperpolarized (HP) 13C-probes.5,6 To expand on the possibilities of HP-MRI modality for ROS sensing, we exploited 15N-labeled boronobenzyl-4-cyanopyridinium (15N-BBCP) as a novel non-invasive imaging probe for detection of H2O2 in vivo. Our preliminary study suggests a promising application of de novo 15N-probe for H2O2 sensing using HP-MRI.Materials and methods
The 15N-BBCP probe was strategically designed to demonstrate desirable HP probe properties. 15N-BBCP was facilely synthesized by conjugation of 4-(hydroxymethyl)phenylboronic ester with 15N-4-cyanopyridine (Figure 1). The ROS selectivity test was conducted by exposing 15N-BBCP to several biologically relevant ROS and monitoring using high-performance liquid chromatography. The H2O2 oxidation reaction kinetics was monitored using UV/Vis spectroscopy. The cytotoxicity assay was performed using Cell Counting Kit-8 (CCK-8) and HeLa cells with various concentrations of BBCP (0–100 mM).3.4M 15N-BBCP samples were prepared in 4:1 DMSO:glycerol with 15mM Ox063 and polarized using a 5T SPINlab (GE Healthcare) for 3-4 hrs. The polarized BBCP samples were dissolved in 6mL dissolution buffer (0.1g/L EDTA, pH = 7.4 in DI water). Spin-lattice relaxation time (T1) of HP 15N-BBCP solution was measured at 1T and 3T using a benchtop 15N NMR spectrometer (Spinsolve; Magritek) and a clinical MRI scanner (Achieva; Philips Healthcare), respectively. A dynamic non-selective pulse-and-acquire sequence with 10o flip angle was used (TR = 10s for 1T, 5s for 3T). The T1 was calculated by fitting the decay curve to a mono exponential function after correcting the RF sampling loss. At 3T, a 15N/1H dual-tuned half-pipe rat birdcage coil (Clinical MR solutions) was used. In vivo studies were performed using a Wistar rat (350 g) at the 3T scanner using the same protocol. The rat received a bolus injection of 20-mM HP 15N-BBCP (4.5mL volume) at baseline.
Results
We confirmed the 15N-BBCP undergoes bioorthogonal and rapid oxidation with H2O2 to unmask phenol, which undergoes concurrent 1,6-elimination to release 15N-4-cyanopyridine. 15N-BBCP showed high selectivity for H2O2 over other biologically relevant ROS. Cytotoxicity assay showed >90% cell viability with 50 mM of 15N-BBCP after up to 24 h of incubation. Kinetics measurements revealed that the reaction between 15N-BBCP and H2O2 proceeds with a second-order rate constant of 8.88±0.55 M-1 min-1 (25 ºC in PBS).The T1 of HP 15N-BBCP solution was measured 340.1s at 1T and 41.2s at 3T. Figure 2A shows the T1 decay of 20-mM HP 15N-BBCP at 3T. Thermally polarized signal was acquired by averaging 600 scans with 90o excitation (Figure 2B). The SNR of HP 15N-BBCP at the first timepoint was 1333 and the polarization level calculated from the HP and thermal scans was estimated as 17.3% at dissolution. In vivo study could detect 15N-BBCP peak at 276 ppm from rat liver (Figure 3).
Discussion/Conclusion
In this study, we designed, synthesized, and applied a novel exogenous 15N-probe for a non-invasive detection of H2O2 using HP 15N MRI. The results show that 15N-BBCP has favorable properties as an HP imaging agent, including high aqueous solubility, low toxicity, long T1 and high ROS sensitivity. These desirable properties of the probe allowed translation of 15N-BBCP to in vivo imaging. Notably, we successfully detected 15N-BBCP in vivo in a normal rat liver. Future studies will test the feasibility of sensing ROS in pathological conditions or detecting its oxidation product in the presence of H2O2. The successful completion of in vivo studies will demonstrate the feasibility of using HP 15N-MRI probes to image and characterize disease environments and monitor therapy progress.Acknowledgements
We acknowledge financial support provided by Duke Cancer Institute, NIH (R21EB024824, P41 EB015908, S10 RR029119, S10 OD018468, R01 NS107409), the Welch Foundation (I-2009-20190330), and the Camille and Henry Dreyfus Foundation.
References
1. Dickinson, B. C., Chang, C. J., Chemistry and biology of reactive oxygen species in signaling or stress responses. Nature Chemical Biology 2011, 7 (8), 504–511.
2. Schieber, M.; Chandel, N. S., ROS function in redox signaling and oxidative stress. Curr Biol 2014, 24 (10), R453–R462.
3. Reth, M., Hydrogen peroxide as second messenger in lymphocyte activation. Nature Immunology 2002, 3 (12), 1129–1134.
4. Parvez, S.; Long, M. J. C.; Poganik, J. R.; Aye, Y., Redox Signaling by Reactive Electrophiles and Oxidants. Chemical Reviews 2018, 118 (18), 8798–8888.
5. Wibowo, A.; Park, J. M.; Liu, S.-C.; Khosla, C.; Spielman, D. M., Real-Time in Vivo Detection of H2O2 Using Hyperpolarized 13C-Thiourea. ACS Chemical Biology 2017, 12 (7), 1737–1742.
6. Lippert, A. R.; Keshari, K. R.; Kurhanewicz, J.; Chang, C. J., A Hydrogen Peroxide-Responsive Hyperpolarized C-13 MRI Contrast Agent. J Am Chem Soc 2011, 133 (11), 3776–3779
Figures
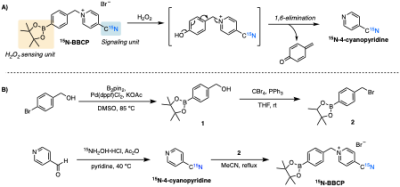
Figure 1. A) Design principles of 15N-BBCP probe for H2O2 sensing. B) Synthetic route to 15N-BBCP
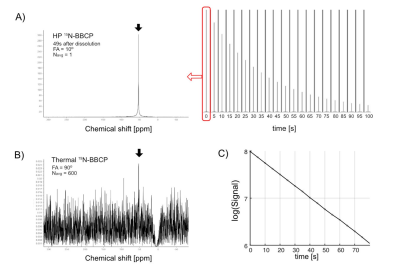
Figure 2. In vitro T1 and polarization level measurement. A) Time series of HP 15N-BBCP at 3T and the first timepoint 15N spectrum. B) Thermal scan of the 15N-BBCP solution. C) Estimation (-1/slope) of the T1 relaxation time.
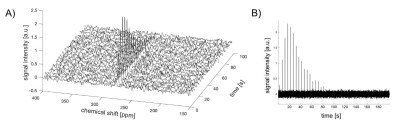
Figure 3. In vivo observation of HP 15N-BBCP in rat liver. A) Stacked plot of 15N spectra acquired from a rat liver at 3T. B) Temporal changes of HP 15N-BBCP peak at 276 ppm.