3286
Validation of non-invasive MR measurement of feto-placental oxygen saturation in a sheep model of human pregnancy1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Department of Medical Physics & Biomedical Engineering, University College London, London, United Kingdom, 3Early Origins of Adult Health Research Group, University of South Australia, Adelaide, Australia, 4Preclinical Imaging and Research Laboratories, South Australian Health and Medical Research Institute, Adelaide, Australia, 5Institute for Women's Health, University College London, London, United Kingdom, 6NIHR Biomedical Research Center, University College London Hospitals, London, United Kingdom
Synopsis
Abnormal placental development is postulated as one of the leading causes of fetal growth restriction (FGR). Advances in MRI technology enable non-invasive measurement of fetal oxygen saturation, but not all have yet been validated. Due to the invasiveness of tests required, validation in human subjects is not possible. Preclinical models such as pregnant sheep allow invasive methods to validate MRI measurements. Here we show that multi-compartment modelling of non-invasive placental MRI can be used to estimate the oxygen saturation of feto-placental oxygenation in normal sheep pregnancy and pregnancies affected by early gestation onstet FGR.
Introduction
Fetal growth restriction (FGR) is a condition in which a fetus has not reached its growth potential1. Management of FGR includes measurement of fetal and maternal uterine artery circulatory resistance from Doppler ultrasound2. However, it is not possible to directly assess fetal oxygenation. Magnetic resonance imaging (MRI) is increasingly providing information on placental function in-vivo to support clinical decision-making. Although no animal model truly recapitulates human pregnancy, pregnant sheep have been used to study maternal-fetal interactions and validate MRI measurements3,4. Non-invasive fetal oxygen saturation (FO2) measurement would be clinically valuable in pregnancies with FGR.The purpose of this study was to: 1) use a placentome-specific multi-compartment MRI model to estimate FO2 using MRI; and 2) validate MRI FO2 against invasively collected blood gas samples.
Methods
The study was approved by the Animal Ethics Committee of the South Australian Health and Medical Research Institute.Carunclectomy surgery
Non-pregnant ewes (n=10) were assigned to have most of their endometrial caruncles removed via carunclectomy5 (CX) under general anesthesia (induction, diazepam (0.3mg/kg) & ketamine (7mg/kg); maintenance, 2.5% isoflurane).
MRI
At 109-111 (Control, n=10; CX, n=10) and 139-141 (Control, n=4; CX, n=5) days gestation, ewes were anesthetised as per surgery and underwent MRI scans on a 3T Siemens Skyra Scanner (Erlangen,Germany). DW-MRI was performed at 7 b‐values (b = 0, 10, 20, 30, 50, 70, 100, 200, 300, 500, 600 s.mm-2) and T2-relaxometry at 10 echo times (TE) = (81,90,96,120,150,180,210,240,270,300 ms). Data was also acquired at b‐value 50 s.mm-2 and 200 s.mm-2 for TE = (81, 90, 120, 150, 180, 210, 240 ms).
Signal-Model
The sheep-specific signal model is of the form:
$$S({\bf b},{\bf{T_E}})= S_0 \left[ e^{{-\bf b}d^*} \Big( f e^{-{\bf {T_E}}R_2^{f_b}}+ v e^{-{\bf {T_E}}R_2^{m_b}}\Big) + (1-f-v)e^{{-\bf b}d-{\bf {T_E}} R_2^{ts}} \right], \quad (1)$$
where $$$S$$$ is the measured MR signal and $$$S_0$$$ is the signal with $$$b=0$$$. The five model parameters are the feto-placenta blood volume fraction $$$f$$$, trophoblast diffusivity $$$d$$$, pseudo-diffusivity $$$d^*$$$, feto-placental blood relaxation $$$R_2^{f_b}=1/T_2^{f_b}$$$ and maternal blood volume fraction $$$v$$$. We used literature-based values for maternal blood relaxation $$$R_2^{m_b}=(150ms)^{-1}$$$ and tissue relaxation $$$R_2^{ts}=(46ms)^{-1}$$$.
Feto-placental Oxygenation
The dependence of FO2 on the effective $$$T_2^{f_b}$$$ is characterised by the Luz-Meiboom model6:
$$\frac{1}{T_2^{f_b}}= \frac{1}{T_{2,0}} + K_0 \Big( 1- \frac{FO_2}{100\%} \Big) + K_1 \Big( 1-\frac{FO_2}{100\%} \Big)^2 \quad (2),$$
where $$$T_2^{f_b}$$$ is the measured $$$T_2^{f_b}$$$ value of partially oxygenated blood calculated in Eq.(1). $$$T_{2,0}$$$, $$$K_0$$$ and $$$K_1$$$ are held fixed at literature7 values of 148.4ms, 1.4 s-1 and 104.4 s-1, respectively.
Image-Analysis
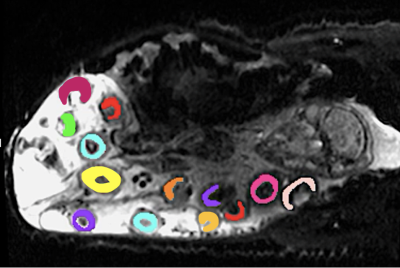
The placentomes where manually segmented from the baseline non-diffusion weighted image. To reduce motion, we applied a non-rigid registration8. Two-way ANOVA was performed to examine the effect of group and gestational age on MRI parameters. Linear regression was performed to compare the relationship between FO2 against reference fetal descending aorta oxygenation measured by catheterisation.
Results
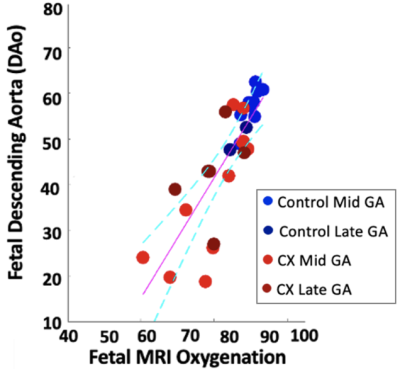
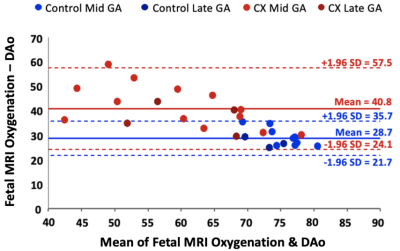
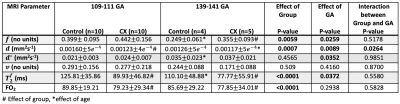
The linear relationship between fetal arterial blood gas and MRI placental based solutions to Luz-Meiboom model was significant for Controls (y=1.64x-91.3, R2 = 0.88, P = 0.0002), for CXs (y = 1.17x - 54.3, R2 = 0.59, P = 0.017) and when all animals were considered together (y = 1.37x - 69.4, R2 = 0.73, P < 0.0001). A strong correlation was observed between fetal DAo measurements and fetal MRI oxygen saturation measurements in Controls (r=0.94, P < 0.0001) and when all animals considered together (r=0.85, P <0.0001); and a medium correlation in CXs (r=0.77, P = 0.0003). Bland Altman analysis showed a mean difference of 28.7% where the limits of agreement ranged from 21.7% to 35.7% for Controls (Figure 3). The mean difference for the CX data was 40.8% and the limits of agreement were wider from 24.1% to 57.5%.We observed a significant decrease in $$$f$$$ between Controls and CX in late gestation (Table 1). $$$T_2^{f_b}$$$ and FO2 were lower in CXs at both gestational ages. $$$d$$$ was lower in CXs, but only in early gestation. $$$d^*$$$ increased with gestation in Controls.
Discussion
We propose a model of separating fetal and maternal circulations to measure oxygen saturation in sheep placentomes. The proposed model measures the oxygenation of fetal blood in the placenta and we show the value is physiologically higher than the sample from the fetal DAo, which is mixed with blood circulating from the fetal body. These differences in feto-placental and DAo saturation result in the consistent bias seen here. We assumed that maternal and fetal blood are intra-capillary but that the maternal blood stays nearly 100% saturated with oxygen. This assumption is not valid, especially in CXs where there is an increased oxygen gradient in the maternal placentome capillaries as a result of the appositional villous structure and is likely also related to the increased bias seen at the lowest oxygen saturations of CXs (Figure 3). Our MRI FO2 results are consistent with what is known about oxygen distribution in fetus9 and show that placental MRI represents a valid means to assess fetal oxygenation status.Conclusion
This study supports the use of multi-compartment placental MRI in pregnancy using sheep as a model of human pregnancy. Results demonstrated a link between MRI FO2 and gold-standard fetal DAo oxygen saturation, supporting the possibility of using MRI for diagnosis of FGR associated with fetal hypoxia.Acknowledgements
This research was supported by the Wellcome Trust (210182/Z/18/Z, 101957/Z/13/Z, 203148/Z/16/Z), the EPSRC (NS/A000027/1) and an ARC Future Fellowship (Level 3; FT170100431) to JLM.References
1. Bullough S, Navaratnam, Sharp A. Investigation and management of the small for gestational age fetus. Obstet Gynaecol Reprod Med. 2021; 31: 1–7.
2. Tay J, Masini G, McEniery CM, Giussani DA, et al. Uterine and fetal placental Doppler indices are associated with maternal cardiovascular function. AJOG. 2019; 220: 96.e1–96.e8.
3. Flouri D, Darby JR, Holman SL, Perumal SR, et al. Magnetic resonance imaging of placentome development in the pregnant ewe. Placental. 2021; 61–69.
4. Morrison JL, Berry MJ, Botting KJ, Darby JRT et al. Improving pregnancy outcomes in humans through studies in sheep. Am J Physiol Regul Integr Comp Physiol. 2018; 315(6): R1123 – R1153.
5. Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response to alpha-adrenergic blockade in fetal sheep during late gestation. J Physiol. 2005; 563:611–620.
6. Wright GA, Hu BS, Macovski A. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5T. J Magn Reson Imaging. 1991; 1: 275–283.
7. Portnoy S, Milligan N, Seed M, et al. Human umbilical cord blood relaxation times and susceptibility at 3 T. Magn Reson Med. 2018; 79: 3194 – 3206.
8. Flouri D, Owen D, Aughwane R, et al. Improved fetal blood oxygenation and placental estimated measurements of diffusion-weighted MRI using data-driven Bayesian modeling. Magn Reson Med. 2020; 83: 2160 – 2172.
9. Saini BS, Darby JR, Portnoy A, Sun L, et al. Normal human and sheep fetal vessel oxygen saturations by T2 magnetic resonance imaging, J. Physiol. 2020; 3259 – 3281.
Figures