3281
An Evaluation of High-Resolution EPI sequences and Enhanced Image Reconstruction for fMRI at 7T: Siemens-EPI, CMRR-EPI and INM4-EPI
Seong Dae Yun1 and N. Jon Shah1,2,3,4
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 3JARA - BRAIN - Translational Medicine, Aachen, Germany, 4Department of Neurology, RWTH Aachen University, Aachen, Germany
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 3JARA - BRAIN - Translational Medicine, Aachen, Germany, 4Department of Neurology, RWTH Aachen University, Aachen, Germany
Synopsis
EPI is of great importance in the MR community due to its wide applicability in various dynamic MR applications. Recent advances in EPI techniques extend its use for the depiction of neuronal activation with a cortical depth-dependence. Therefore, enhanced performance with reliable image quality is highly desirable in EPI. This work presents an in-house designed EPI sequence and its corresponding image reconstruction software at 7T. Results show that our EPI sequence outperforms two widely used sequences: Siemens-EPI and CMRR-EPI. In addition, our image reconstruction routine significantly improves the reconstructed image quality of Siemens and CMRR data.
Introduction
Echo planar imaging (EPI) is widely used in the MR community, and its relatively high temporal resolution makes it particularly adept for numerous dynamic MR applications, such as fMRI or DWI. Recent advances in EPI techniques extend its use for the depiction of neuronal activation with a cortical depth-dependence at ultra-high fields. Therefore, enhanced performance with reliable image quality is highly desirable in EPI. For these applications, a commercial, vendor-supplied, EPI sequence is usually employed. However, for advanced imaging options, which cannot be supported by the commercial EPI sequence, a custom-made EPI sequence is often developed, and, of these, the one from CMRR (Center for Magnetic Resonance Research) is the most widely used.1-4 In this work, we present our own in-house designed EPI sequence (INM4-EPI), developed in a Siemens environment5, and evaluate its performance in comparison to two other widely used EPI sequences: Siemens-EPI and CMRR-EPI. For this purpose, this work configures several imaging protocols at 7T and demonstrates the improved performance offered by our in-house designed sequence and online image reconstruction software.Methods
The in-house designed EPI sequence was developed using the standard Siemens sequence development platform, IDEA (Integrated Development Environment for Applications) VE12U. The development was based on the manual implementation of all of the sequence components necessary for 2D gradient-echo EPI, but without the Siemens EPI sequence as a basis template code. The sequence was designed to support the combination of parallel imaging, partial Fourier and multi-band techniques, in which the data reconstruction for the first two techniques was handled by the default reconstruction routine from Siemens. The reconstruction of the multi-band technique was performed by our in-house implemented ‘Slice-GRAPPA’ method,6 which was subsequently integrated into the default Siemens reconstruction routine, i.e. ICE (Image Calculation Environment). The performance of the three EPI sequences (Siemens, CMRR: version R016 and INM4) was evaluated in a typical fMRI setting: TR = 2500 ms, FA = 90°, FOV = 210 × 210 mm2 and sequence type = 2D gradient-echo. Various imaging protocols were configured with a number of matrix sizes and acceleration conditions (Fig. 1). Data sets from a uniform spherical phantom and two healthy subjects, screened with a standard safety procedure, were acquired at a Siemens Magnetom Terra 7T scanner with a 1-Tx/32-Rx head coil. The performance difference of the three sequences was investigated by applying our in-house implemented reconstruction routine to the Siemens and CMRR data. Finally, the image reconstruction times of the three EPI sequences were compared.Results
Figure 2 shows the reconstructed phantom images obtained from four accelerated protocols. The figure shows that for all resolution cases, aliasing artefacts arising from the acceleration techniques were clearly eliminated in the INM4 data, whereas a significant amount remain for the Siemens and CMRR data, causing substantial signal loss. The degree of aliasing artefacts in these two sequences was much greater when a larger parallel imaging factor was applied (i.e. 2nd row; three-fold) than a smaller one (i.e. 1st raw; two-fold). Here, particularly for the ‘Protocol D’ (1.0 mm), which was shown to be the worst-case in terms of the aliasing artefacts, the Siemens and CMRR data were reconstructed with our in-house implemented image reconstruction function. As a result, the aliasing artefacts observed in the two sequences were significantly reduced in the new reconstructed images (3rd and 4th columns in Fig. 3) and were observed to be very comparable to the image from INM4-EPI (5th column in Fig. 3). Figure 4a shows the reconstructed in vivo images obtained from ‘Protocol D’ (1.0 mm). Similar to the phantom case, aliasing artefacts or signal loss can be observed in the Siemens and CMRR data around the regions marked by the yellow arrows. Figure 4b shows in vivo data from another subject, demonstrating that the signal loss observed in the Siemens and CMRR data was significantly recovered by means of our image reconstruction routine. Figure 5 shows the image reconstruction time of the three sequences, measured for the following data sets: ‘Protocol A’ (1.6 mm), multi-band factors of 1, 2, …, 8 and 50 temporal volumes. Here, the corresponding images are shown alongside. The graph demonstrates that Siemens-EPI had the fastest reconstruction performance. However, the reconstruction time of INM4-EPI becomes comparable to Siemens-EPI as the multi-band factor increases; for a multi-band factor of 8, the reconstruction times of INM4-EPI and Siemens-EPI are very similar each other.Discussion and conclusions
This work demonstrates the superior performance of our in-house designed EPI sequence (INM4-EPI) compared to two other widely employed EPI sequences: Siemens-EPI and CMRR-EPI. Results from the Siemens and CMRR data show that the degree of aliasing artefacts became greater for relatively large parallel imaging and multi-band acceleration conditions. However, these aliasing artefacts were effectively eliminated by our in-house implemented image reconstruction routine. Furthermore, our image reconstruction software showed image reconstruction times comparable to the Siemens case. It is expected that the improved performance demonstrated with our 2D GE-EPI in this work can be directly transferred to other types of EPI (e.g 2D diffusion-EPI, 2D SE-EPI, etc.). Additionally, future work will attempt to repeat these investigations on other 7T scanners to determine if the improvements are of general validity.Acknowledgements
No acknowledgement found.References
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, et al. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magnetic Resonance in Medicine. 2010;63(5):1144-53.
- Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010;5(12):e15710.
- Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, et al. Evaluation of slice accelerations using multiband echo planar imaging at 3T. NeuroImage. 2013;83:991-1001.
- Available at http://www.humanconnectomeproject.org.
- Available at https://www.siemens-healthineers.com/magnetic-resonance-imaging/7t-mri-scanner/magnetom-terra.
- Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012 May;67(5):1210-24.
Figures
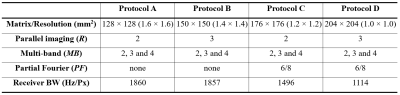
Figure 1. Four accelerated imaging protocols
used for the assessment of three EPI sequences. Each imaging protocol was
configured with the following condition: TR = 2500 ms, FA = 90°, FOV = 210 × 210 mm2
and sequence type = 2D gradient-echo.
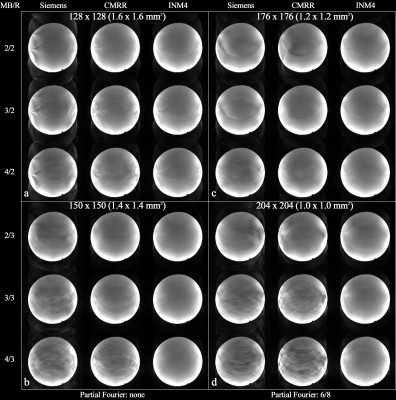
Figure 2. Reconstructed images obtained with
the protocols in Fig. 1. Each image panel (a – d) shows results obtained with the
Siemens, CMRR and INM4 EPI sequences. The applied multi-band and parallel
imaging acceleration factors are shown on the left side, denoted by ‘MB/R’. For
the relatively higher resolution cases (i.e. c and d), a partial Fourier factor
of 6/8 was applied. The data from Siemens and CMRR show increases in aliasing
artefacts for a larger multi-band factor, whereas the aliasing artefacts are
more clearly eliminated in the INM4 data.
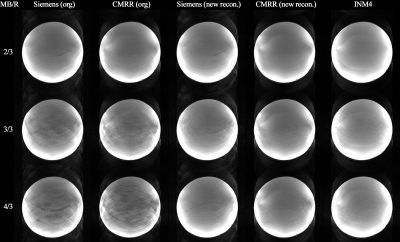
Figure 3. Results of the retrospective
reconstruction of Siemens and CMRR data using our in-house implemented
reconstruction routine. The labels ‘org’ and ‘new recon.’ indicate cases where
the original image reconstruction and our image reconstruction routines were
applied, respectively. A significant reduction in aliasing artefacts can be
observed in the ‘new recon.’ images of Siemens and CMRR data when compared to
the ‘org’ images. The above reconstruction was demonstrated with the data
obtained with the ‘Protocol D’ (1.0 mm).
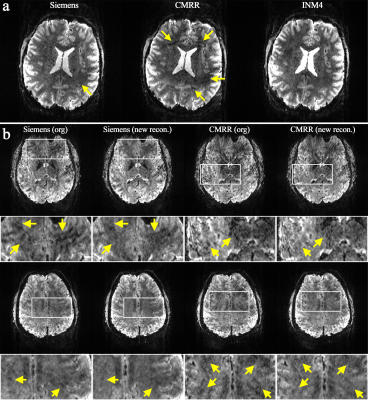
Figure 4. Reconstructed in vivo slices obtained from the ‘Protocol D’ (1.0 mm). (a) A reconstructed slice showing increased aliasing artefacts or signal loss in the Siemens and CMRR data (see the yellow arrows) when compared to INM4 data. (b) Results of the retrospective reconstruction of Siemens and CMRR data using our in-house implemented reconstruction routine. The signal loss observed in the original images was significantly recovered in the ‘new recon’ images. An ROI (see white squares) was selected for each slice, and its magnified view is depicted below.
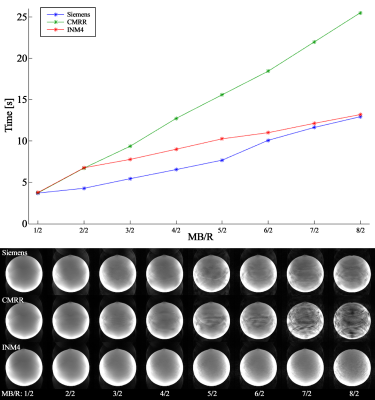
Figure 5. (Top) Image reconstruction times
of the three EPI sequences, measured for the data acquired with the following
option: ‘Protocol A’ (1.6 mm), multi-band factors = 1, 2, …, 8 with an
increment of 1, number of effective RF excitations for each multi-band factor =
4, temporal volumes = 50. (Bottom) Corresponding reconstructed images of the
three sequences. Although Siemens showed the fastest performance for all
multi-band acceleration, i.e. MB ≥ 2, the reconstruction time from INM4 becomes
comparable to Siemens as the multi-band factor increases.
DOI: https://doi.org/10.58530/2022/3281