3278
Using amide proton transfer-weighted (APTw) MRI to detect severity and predict outcome after traumatic brain injury (TBI) in rats1Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University, Baltimore, MD, United States, 2Department of Radiology, Johns Hopkins University, Baltimore, MD, United States
Synopsis
After TBI, secondary injury severity is difficult to determine. The objective of this study was to investigate the capacity of noninvasive APTw MRI to assess TBI injury in different brain regions and predict long-term neurobehavior outcomes. Fifty-five male and female rats were subjected to a controlled cortical impact with one of three different impactor depths to produce different degrees of TBI, and scanned on a 4.7 T horizontal bore animal imager. Our results suggest that APTw imaging can be used for detecting the level of inflammation and as a potential predictor of long-term outcomes from TBI.
Introduction
Neuroimaging is an important tool in the diagnosis and characterization of TBI and the prediction of outcome, but more sensitive and reliable techniques are needed.1 APTw imaging is a novel molecular MRI technique that is sensitive tissue pH and the concentration of endogenous mobile proteins and peptides.2 Prior studies have indicated that protein-based APTw MRI is a sensitive marker for acute identification of neuroinflammation in TBI.3 However, TBI outcome depends on the injury severity and the particular brain regions that are damaged. In this study, we used multi-parameter MRI, including APTw, to determine spatial and temporal changes that occur within the first 3 days after a controlled cortical impact (CCI) of different severities in rats.Methods
AnimalsFifty-five adult male (n=29) and female (n=26) Sprague-Dawley rats (240-300 g) were randomly divided into four groups (sham, mild TBI, moderate TBI, and severe TBI). To produce CCI, the bone flap was removed and the dura was impacted with a benchmark stereotaxic impactor with a diameter of 3 mm at a velocity of 5.5 m/s and a dwell time of 100 ms. To produce a broad range of injury for regression analysis with APTw, the impact depths was varied over a wide range (1, 3, or 5 mm), corresponding to mild, moderate and severe injury, respectively. Rats in the sham group were subjected to anesthesia and a scalp incision but did not undergo a craniotomy or impact.
MRI data acquisition and analysis
MRI data were acquired at 1 h, 1 day, and 3 days on a 4.7 T horizontal bore animal imager (Bruker Biospin; Billerica, MA). The multiparametric MRI protocol used in this study included coronal T2w, T1w, and T2*w sequences, and six quantitative or semiquantitative MRI sequences were acquired, including T2, T1, isotropic ADC, CBF, APTw (saturation power = 1.3 μT; saturation time = 4 sec), and MTR @ 10 ppm or 2 kHz at 4.7 T. Data were processed with IDL. Specially, the APTw images were calculated based on the MTR asymmetry at ±3.5 ppm. ROIs were drawn manually for quantitative analysis, using T2w and MTR as a reference, and then transferred to identical sites on all other co-registered MRI maps.
Behavioral tests and immunofluorescence staining
Several neurobehavioral tests (neurologic severity score, Barnes maze, sucrose preference test, forced swim test, immunofluorescence staining) over a 1-month recovery period were performed, and immunofluorescence was also performed to assess microglial cell density at day 3 post-TBI.
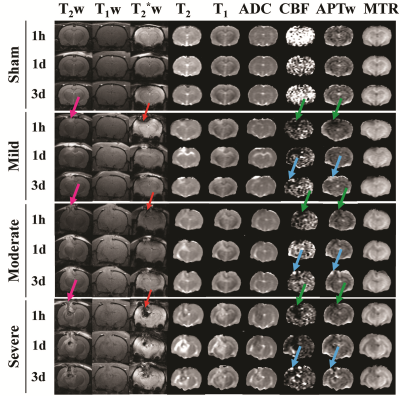
Results
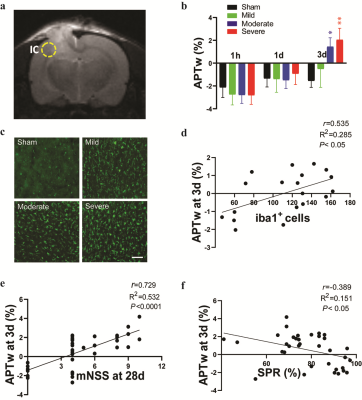
Fig. 1 shows the time course of multiparametric MRI features of CCI rats. High-resolution MRI images (T2w and T2*w) showed primary injury (such as hemorrhage) on the impacted site. At 1 h post-CCI, all CCI groups exhibited changes in MRI features (increases or decreases), compared to those of the sham group. Specifically, CBF and APTw were considerably reduced in the core, perilesional cortex, and other ipsilateral areas. By day 3 after CCI, the CBF and APTw remained largely decreased in the core. However, APTw and CBF hyperintensities were clearly visible in most ipsilateral areas. Thus, different MRI signals have unique responses to TBI that vary with ROI, likely reflecting different aspects of dynamic pathophysiologic processes occurring across brain regions.Fig. 2 shows the correlation of APTw signal in perilesion cortex with neuroinflammation and long-term outcomes after TBI. The APTw signal in the perilesional cortex lateral to the core injury gradually increased after CCI relative to that in the sham group, with significant increases in the moderate and severe groups at 3 days (Fig. 2b). The number of Iba1-positive cells was markedly higher in the perilesion cortex of the CCI groups than in that of the sham group at 3 days (Fig. 2c) and showed a positive correlation with the APTw signal by Pearson’s correlation analysis (Fig. 2d). Furthermore, APTw at 3 days positively correlated with the mNSS and negatively correlated with the sucrose preference rate (SPR) 1 month after CCI (Fig. 2e, f). These data indicate that the APTw signal in the perilesion cortex at 3 days post-TBI is closely related to the neuroinflammatory response and associated with long-term neurologic dysfunction and anhedonia.
Discussion
Accumulating evidence indicates that neuroinflammation is critically important to secondary injury and influences long-term neurologic deficits after TBI.4, 5 Here, we showed that the magnitude of the increase in APTw signal in the perilesion cortex at 3 days post-TBI above that in the sham group depended on the injury severity and, interestingly, correlated with the number of activated microglia, which release a variety of cytokines in the subacute stage of TBI.6, 7 These results suggest that increased APTw signal in the perilesion cortex at 3 days may result from an increase in cellular proteins associated with the inflammatory process. Because the neuroinflammatory response affects long-term behavior and the APTw signal is associated with microglial activation, the APTw signal at 3 days appears to provide prognostic information about the long-term neurobehavior outcomes after TBI.Conclusion
Our study illustrates the potential for APT imaging to assess the severity of injury and the level of inflammation at the acute stage of TBI. This noninvasive MRI may be useful for stratifying patients in clinical trials and treatment regimens.Acknowledgements
This work was supported in part by a grant from the National Institutes of Health (UH3NS106937).References
1. Amyot F, Arciniegas DB, Brazaitis MP, Curley KC, Diaz-Arrastia R, Gandjbakhche A, Herscovitch P, Hinds SR, 2nd, Manley GT, Pacifico A, Razumovsky A, Riley J, Salzer W, Shih R, Smirniotopoulos JG, Stocker D. A Review of the Effectiveness of Neuroimaging Modalities for the Detection of Traumatic Brain Injury. J Neurotrauma. 2015;32:1693-1721.
2. Zhou J, Heo HY, Knutsson L, van Zijl PCM, Jiang S. APT-weighted MRI: Techniques, current neuro applications, and challenging issues. J Magn Reson Imaging. 2019;50:347-364.
3. Zhang H, Wang W, Jiang S, Zhang Y, Heo HY, Wang X, Peng Y, Wang J, Zhou J. Amide proton transfer-weighted MRI detection of traumatic brain injury in rats. J Cereb Blood Flow Metab. 2017;37:3422-3432.
4. Willis EF, MacDonald KPA, Nguyen QH, Garrido AL, Gillespie ER, Harley SBR, Bartlett PF, Schroder WA, Yates AG, Anthony DC, Rose-John S, Ruitenberg MJ, Vukovic J. Repopulating Microglia Promote Brain Repair in an IL-6-Dependent Manner. Cell. 2020;180:833-846 e816.
5. Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, Szeto GL, Wu J, Stoica BA, Faden AI, Loane DJ. Microglial Depletion with CSF1R Inhibitor During Chronic Phase of Experimental Traumatic Brain Injury Reduces Neurodegeneration and Neurological Deficits. J Neurosci. 2020;40:2960-2974.
6. Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation. 2017;14:47.
7. Witcher KG, Bray CE, Chunchai T, Zhao F, O'Neil SM, Gordillo AJ, Campbell WA, McKim DB, Liu X, Dziabis JE, Quan N, Eiferman DS, Fischer AJ, Kokiko-Cochran ON, Askwith C, Godbout JP. Traumatic Brain Injury Causes Chronic Cortical Inflammation and Neuronal Dysfunction Mediated by Microglia. J Neurosci. 2021;41:1597-1616.
Figures