3277
Minimally-Invasive Surgical Treatment of Lung Cancer using Magnetic Resonance Guided Focused Ultrasound in a Porcine Model1University of Virginia, Charlottesville, VA, United States, 2Focused Ultrasound Foundation, Charlottesville, VA, United States
Synopsis
This new technique has the potential to increase lung cancer survival rates while decreasing morbidity associated with current practices as it allows a less invasive manner for ablation or tumor debulking.
Introduction
Lung cancer is the second most commonly diagnosed cancer amongst men and the third most commonly amongst women and is the leading cause of cancer related deaths in both sexes with an estimated 142,080 deaths in 2018.1 Treatment strategy for non-small cell lung cancer (NSCLC) depends on clinical staging, but surgical lobectomy is the primary treatment for stage I and II cancers while also being an accepted treatment for stage III and IV disease. Surgery may be a curative treatment in stage I NSCLC, and patients with stage 1 cancers treated with surgery have a five-year survival rate of 43%-73% compared to a five-year survival rate of 6% for untreated patients2. Unfortunately, 20% of patients with stage I/II NSCLC are not eligible for lobectomy, and for those undergoing surgery there is a 1-5% incidence of operative mortality.3 This project proposes an innovative, minimally invasive procedure for the transcutaneous ablation and subsequent complete removal or significant reduction of lung cancer masses using Magnetic Resonance Guided Focused Ultrasound (MRgFUS). This technique does not require flooding of the lung to gain an acoustic window for Focused Ultrasound (FUS) which reduces the risk of immediate and long term risks to the patient including pneumonia and sepsis. A previous study demonstrated the feasibility of MRgFUS in the ablation of malignant pleural mesothelioma where this technique did not require the incisions but required longer time to treat large areas when compared to radiofrequency ablation.4Materials & Methods
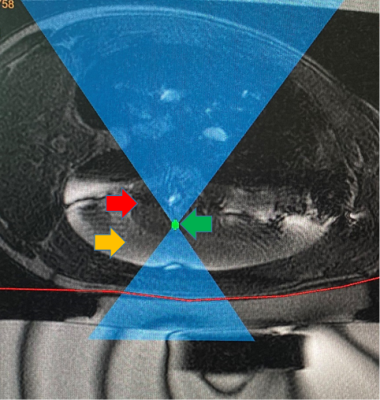
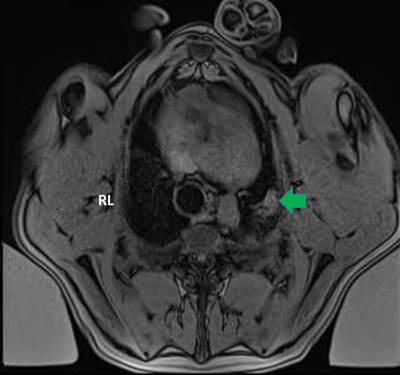
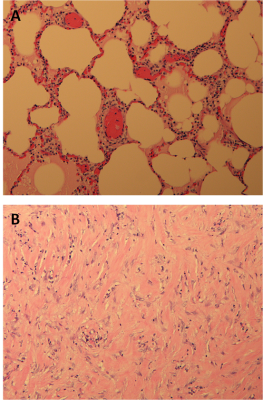
Seven Yorkshire pigs (53-63Kg) had both main bronchi selectively intubated, and while the right lung was mechanically ventilated, the left lung was purposely collapsed, followed by a controlled left hydrothorax in the chest cavity with saline (1000cc) via a 2cm incision in order to create an acoustic window for the FUS beam. Animals received two or three transcutaneous MRgFUS ablations of the left lung, in two or three different locations, Figure 1. Each location received a different amount of acoustic power in order to determine the optimal power with minimal side effects (i.e. skin burns), Table 1. An intercostal approach was used in order to target the transcutaneous beam with minimal distortion and attenuation caused by the ribs. Ablations were performed at maximum inhalation of the non-dependent lung (right lung). At the end of the MRgFUS ablation, Thoracentesis was performed and saline was drained in a method identical to the drainage of a clinical pleural effusion. All pigs remained stable for one week after the ablation, at that time each animal received a complete MRI of the lung using T2w, and T1w sequences both pre and post contrast (10mL Magnevist/Gadavist) to assess the success of the ablation, Figure 2. Pigs were then euthanized followed by histopathologic examination to correlate with the results of the MRI, Figure 3.Results
Ablations were performed successfully in each animal, and there were areas of hyperintensity on T1 MRI corresponding to areas targeted for ablation. Locations and intensity of ablations confirmed by MRI and gross histology aligned well with target locations and applied powers. In all studies the heart and pericardium were without lesions. Pig 1, had a small area of a skin burn but it was remedied in later studies by using a thinner gel pad under the pig allowing the cooling table to have more cutaneous effect; Pigs 2-7 showed no evidence of first degree burns at the areas of entry of FUS beams. In Pig 2 there was damage to the subcutaneous tissues in the FUS path. In Pig 3 there was a small pneumothorax and in addition to the three ablated spots which were firm, the lung parenchyma had a gelatinous texture. Pigs 2, 3, 4 and 6 presented with firm, nodular lesions in at the areas of ablation. Pig 5 presented with a unique 3cm cystic lesion filled with pus over the area of ablation. Pig 7 presented with a small lesion in the right lung possibly caused by reflection of the MRgFUS beam. All pigs remained healthy in the week post ablation.Discussion
These results are very encouraging as they demonstrate the ability for transcutaneous MRgFUS ablation in-vivo to induce damage of lung parenchyma behind the rib space. A lot was learned but more work needs to be done to perfect this technique and assess tissue necrosis in cancer tumors. Each animal model recovered well from this surgery with no poor outcomes. This technique also opens the possibility for intrathoracic delivery of ultrasound for lung cancer tumors that are difficult to reach using an intercostal approach. A therapeutic use for high intensity focused ultrasound (HiFU) has been demonstrated in ex-vivo human cancer models using one-lung flooding (OLF) and in mice models against cisplatin-resistant human lung adenocarcinoma.5,6 The risks of OLF in patients with reduced lung function are still in question however.5Conclusion
This new technique has the potential to increase lung cancer survival rates while decreasing morbidity associated with current practices as it allows a less invasive manner for ablation or tumor debulking. For the large population who previously could not tolerate resection, radiotherapy, or chemotherapy this could serve as a treatment where previously only palliative care may have been an option.Acknowledgements
Work funded by a grant from the Focused Ultrasound Foundation.References
1) U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool (1999–2018): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, June 2021.
2) Hoy H, Lynch T, Beck M. Surgical Treatment of Lung Cancer. Critical Care Nursing Clinics of North America. 2019;31(3):303-313. doi:https://doi.org/10.1016/j.cnc.2019.05.002
3) Mehta HJ, Ross C, Silvestri GA, Decker RH. Evaluation and treatment of high-risk patients with early-stage lung cancer. Clin Chest Med. 2011;32(4):783-797 doi:10.1016/j.ccm.2011.08.011
4) Costa M, et al. Cytoreductive Surgical Treatment of Pleural Mesothelioma: A Comparison Study Between Radiofrequency Ablation and MRgFUS Treatments in a Swine Model of Mesothelioma. International Society for Magnetic Resonance in Medicine; April 2013, Salt Lake City.
5) Wolfram F, Boltze C, Schubert H, Bischoff S, Lesser TG. Effect of lung flooding and high-intensity focused ultrasound on lung tumours: an experimental study in an ex vivo human cancer model and simulated in vivo tumours in pigs. Eur J Med Res. 2014;19(1):1. Published 2014 Jan 7. doi:10.1186/2047-783X-19-1
6) Zhang T, Chen L, Zhang S, Xu Y, Fan Y, Zhang L. Effects of high-intensity focused ultrasound on cisplatin-resistant human lung adenocarcinoma in vitro and in vivo. Acta Biochim Biophys Sin (Shanghai). 2017;49(12):1092-1098. doi:10.1093/abbs/gmx10
Figures