3274
VEGF overexpression in breast cancer xenograft significantly increases nanoparticle-mediated siRNA delivery and target gene downregulation1The Russell H Morgan Department of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States, 2Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD, United States, 3Department of Radiation Oncology and Molecular Radiation Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
Effective tumor delivery is a major challenge in achieving small interfering RNA (siRNA) mediated gene silencing. Poor tumor vascularization can limit the delivery of therapeutic siRNA. Here, we used a VEGF overexpression breast cancer murine model and its wildtype counterpart to investigate the influence of increasing tumor vasculature on choline kinase-alpha siRNA nanoparticle delivery.
Introduction
As gene-silencing agents, siRNA have significantly expanded the specificity and range of ‘druggable’ targets making them promising agents for precision medicine in cancer1. Effective delivery and cellular uptake, the major challenges in siRNA nanoparticle (NP) therapeutics, become even more challenging in cancer because of the complexities of the tumor microenvironment (TME) such as the abnormal vasculature. Studies directly relating NP delivery to function are necessary to understand how these NPs navigate through the TME, and to understand the impact of the TME on siRNA delivery and function2. Here we used triple negative MDA-MB-231 human breast cancer xenografts derived from cells engineered to overexpress VEGF to understand the role of VEGF and vascularization in NP delivery and function. VEGF overexpression increases vascularization in these tumors3. We used polyethylenimine (PEI) NPs to deliver siRNA to downregulate choline kinase-alpha (Chk-a), an enzyme that is associated with malignant transformation and tumor progression4. Chk-α converts choline to phosphocholine (PC), a key precursor of membrane phospholipids.Methods
Tumors were obtained by inoculating 1×106 MDA-MB-231 wild type cells, or MDA-MB-231 cells engineered to stably express VEGF, orthotopically in female SCID mice. PEI NPs were labeled with Cy5.5 dye for optical imaging with a Pearl® Trilogy Small Animal Imaging System (LI-COR, Lincoln, NE). PEI NPs delivering Chk-a siRNA were administered intravenously with two doses of 3 nmol Chk-a siRNA given 24h apart, once tumors were ~ 300-400 mm3. In vivo and ex vivo optical imaging was used to track NP delivery and distribution in 6 wild type and 6 VEGF overexpressing tumors at 24h following injection of the second dose of siRNA NPs. RT-PCR and immunoblotting were used to evaluate Chk-α levels in tumors excised at 24h after the injection of second dose. High-resolution 1H MRS of tumor extracts at 11.7 T was used to evaluate changes in choline, phosphocholine, and glycerophosphocholine (GPC).Results and Discussion
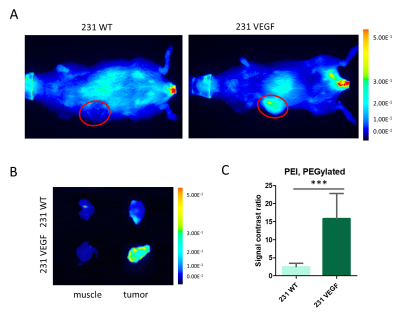
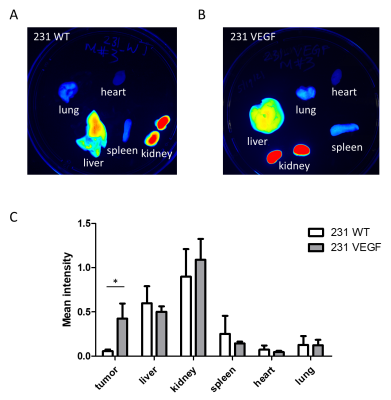
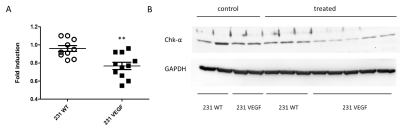
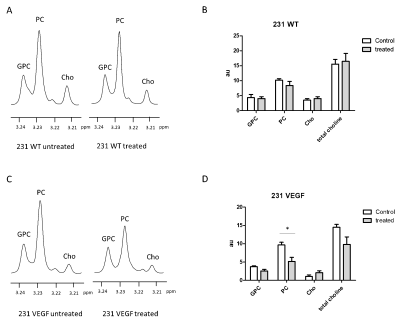
Significantly higher Cy5.5 fluorescence intensity was observed in VEGF overexpressing MDA-MB-231 tumors compared to wild type MDA-MB-231 tumors as shown in the representative in vivo (Fig. 1A) and ex vivo (Fig. 1B) images. Analysis of the tumor/muscle ratio demonstrated a significant increase of the intensity ratio in VEGF overexpressing tumors (Fig. 1C). Representative ex vivo images of Cy5.5 intensities in organs, summarized in Fig. 2, demonstrated that VEGF overexpression in tumors did not alter PEI NP biodistribution in the other organs including the liver, kidney, lung, heart, and spleen. RT-PCR (Fig. 3A) and immunoblots (Fig. 3B) analysis of tumors confirmed that the improved NP delivery in VEGF overexpressing tumors resulted in a significantly improved downregulation of Chk-a mRNA and protein. High-resolution 1H MRS of tumor extracts showed siRNA NP treatment did not significantly reduce phosphocholine in MDA-MB-231 tumors (Fig. 4A, B), whereas a significant decrease of phosphocholine was observed in MDA-MB-231-VEGF tumors (Fig. 4C, D). These data demonstrate the importance of vascular delivery in achieving successful target downregulation and highlight the role of imaging in evaluating NP delivery. Our data indicate that strategies to improve vascular delivery of NPs in tumors are important in the use of siRNA to achieve gene-specific downregulation.Acknowledgements
Funding support: NIH R01 CA253617 and R35 CA209960References
1. Chen, Z.; Krishnamachary, B.; Pachecho-Torres, J.; Penet, M. F.; Bhujwalla, Z. M., Theranostic small interfering RNA nanoparticles in cancer precision nanomedicine. Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology 2019, e1595.
2. Danhier, F., To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? Journal of controlled release : official journal of the Controlled Release Society 2016, 244 (Pt A), 108-121.
3. Pathak, A. P.; McNutt, S.; Shah, T.; Wildes, F.; Raman, V.; Bhujwalla, Z. M., In vivo "MRI phenotyping" reveals changes in extracellular matrix transport and vascularization that mediate VEGF-driven increase in breast cancer metastasis. PloS one 2013, 8 (5), e63146.
4. Glunde, K.; Bhujwalla, Z. M.; Ronen, S. M., Choline metabolism in malignant transformation. Nat Rev Cancer 2011, 11 (12), 835-48.
Figures