3273
Early sex dependent changes in the default mode network of the TgF344-AD rat model of Alzheimer’s disease1Biomedical Imaging Group, Consorcio Centro de Investigación Biomédica en Red (CIBER) de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Barcelona, Spain, 2Experimental 7T MRI Unit, Magnetic Resonance Imaging Core Facility, Institut d’Investigacions Biomèdiques August Pi I Sunyer (IDIBAPS), Barcelona, Spain, 3Laboratory of Surgical Neuroanatomy, Faculty of Medicine and Health Sciences, Institute of Neurosciences, University of Barcelona, Barcelona, Spain
Synopsis
TgF344-AD rat model has been revealed as an interesting animal model for investigating Alzheimer's disease. About two thirds of persons diagnosed with AD dementia are women, suggesting important sex and gender differences in risk factors for the development of AD. Therefore, it is of high interest to also investigate this issue in the AD animal models. In this study we present preliminary results regarding the effect of sex difference in the functional default mode network between young TgF344-AD rats and Wild-type.
Introduction
TgF344-AD rat model (TG) has been revealed as an interesting animal model for investigating Alzheimer's disease (AD). We have recently demonstrated dysfunction in several resting state networks such as the default mode in male TG rats at 5-6 months of age1. About two thirds of persons diagnosed with AD dementia are women, suggesting important sex and gender differences in risk factors for the development of AD2. Therefore, it is of high interest to also investigate this issue in the AD animal models. In this line, the aim of this study was to study the effect of sex in the default mode network of young wild-type (WT) and TG rats.In this study we present preliminary results regarding the sex-dependent early differences in functional default mode network between young TgF344-AD rats and Wild-type.
Methods
The experimental groups were: WT male n=24; WT female n=14; TG male n=18; TG female n=13. The animals were 3.1 ± 0.1 months of age at the time of the scan without significant differences between the four groups.Resting state fMRI was acquired in a 7T Bruker scanner by using a single-shot gradient-echo EPI sequence. Images had 600 volumes of 64x64x34 voxels and 0.4x0.4x0.6 mm³/voxel with TR=2s and TE=28ms.
Image preprocessing included: slice-timing, motion correction, skull-stripping, spatial normalization, spatial smoothing, detrending and regression by motion parameters, and temporal filtering (0.01-0.1Hz). 30 independent components were obtained using FSL MELODIC3 considering the whole cohort and identifying the DMN between the resulting components (Figure 1).
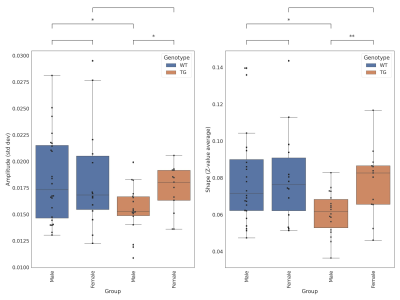
The Amplitude -as the standard deviation of the time-series obtained from the first stage of the dual regression- and the Shape -as the mean of the z-values within the thresholded (z > 2.3) spatial map obtained from the second stage of the dual regression- were computed for each subject and the differences between groups were evaluated using Kruskall-Wallis test.
Results
The amplitude and shape, as measures of the magnitude and location of the BOLD activity, in the default mode network were significantly decreased in the TG male group compared to the WT male group (p<0.05), but no significant differences were observed between female TG and WT groups. This results in significant differences between TG female and TG male groups (p<0.05), while this gender difference was not observed in the respective WT groups (Figure 2). In addition, TG male rats showed a significant decrease of the two measures compared to the WT male group, a difference that was not observed between the female groups.Discusion
In a previous study, alterations in the anterior DMN subnetwork activity of male TG rats compared to controls were observed at 5 months of age1. Other authors using the same TG rats found slightly decreased functional connectivity at 6 months followed by severe and widespread hypoconnectivity at 10 months of age in female TG rats compared to wild-type (WT) animals4. In the present study, we evaluated this model at earlier ages (3 months of age) and used male and female WT and TG littermates paired by age to investigate in the optimal experimental conditions the effect of sex in early alterations of functional resting state networks induced by the double mutation of mutant human amyloid precursor protein (APPswe) and Presenilin 1 (PSEN1ΔE9) genes. Our preliminary results show decreased functional connectivity measurements in the TG male rats with respect to WT at very early and points to sexual differences in these early alterations of DMN in TG male animals compared to TG female rats.Conclusion
In this work we have seen significant differences in the amplitude and shape of the default mode network in male TG rats compared to female TG rats and to male WT rats pointing to functional sex differences at early ages in these rats. This support the idea that research is required in both males and females to obtain the whole picture of pathological processes in animal models.Acknowledgements
This work has been funded by the project PI18/00893, integrated in the Plan Nacional I+D+I and co-funded by ISCIII-Subdirección General de Evaluación and European Regional Development Fund (ERDF) "A way to make Europe" and Secretaria d’Universitats i Recerca del Departament d’Empresa I Coneixement de la Generalitat de Catalunya AGAUR 2017 SGR 01003. CIBER-BBN is an initiative financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund. We are indebted to the Experimental MRI 7T Unit of the IDIBAPS.References
1. Tudela R, Muñoz-Moreno E, Sala-Llonch R, López-Gil X, Soria G. Resting State Networks in the TgF344-AD Rat Model of Alzheimer's Disease Are Altered From Early Stages. Front Aging Neurosci. 2019 Aug 8;11:213
2. Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, Mallampalli MP, Mormino EC, Scott L, Yu WH, Maki PM, Mielke MM. Understanding the impact of sex and gender in Alzheimer's disease: A call to action. Alzheimers Dement. 2018 Sep;14(9):1171-1183.
3. Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005 Mar;25(1):294-311.
4. Anckaerts C, Blockx I, Summer P, Michael J, Hamaide J, Kreutzer C, Boutin H, Couillard-Després S, Verhoye M, Van der Linden A. Early functional connectivity deficits and progressive microstructural alterations in the TgF344-AD rat model of Alzheimer's Disease: A longitudinal MRI study. Neurobiol Dis. 2019 Apr;124:93-107.
Figures