3272
Longitudinal biochemical and behavioral alterations in a gyrencephalic model of blast related mild traumatic brain injury1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Center for Advanced Imaging Research (CAIR), University of Maryland School of Medicine, Baltimore, MD, United States, 3Blast Induced Neurotrauma Branch, Walter Reed Army Institute of Research, Silver Spring, MD, United States, 4Department of Pediatrics, University of Maryland School of Medicine, Baltimore, MD, United States, 5Department of Anesthesiology, University of Maryland School of Medicine, Baltimore, MD, United States, 6Shock, Trauma, and Anesthesiology Research Center, University of Maryland School of Medicine, Baltimore, MD, United States
Synopsis
Compared to rodents, the ferret model has greater similarities to humans in terms of developmental process, brain structure and sophisticated behavior. In this study, we assessed the longitudinal changes in brain metabolism and impulsivity behavior in a ferret model that closely mimics the blast exposure conditions encountered by Warfighters. Ferrets demonstrated concomitantly increased behavioral impulsivity and metabolite alterations in prefrontal cortex following blast exposure. Our findings agree with clinical observations in patients, suggesting that this model is a good gyrencephalic animal model to study brain biochemical profile changes and neuropsychiatric alterations associated with blast exposure.
Introduction
Much of military traumatic brain injury (TBI) occurs in the form of blast overpressure (BOP)-related injuries. Among these types of injuries, the long-term sequelae from BOP exposure are perhaps the least understood primarily because of the variability and heterogeneity of the BOP exposure leading to complex debilitating long-term outcomes in humans. Animal models can complement clinical studies and contribute to our understanding of the neurobiological underpinnings BOP TBI that could provide insights into pathogenesis in a more controllable and efficient way.To understand effects of BOP, we used a gyrencephalic model of blast (ferret) which underwent blast exposure that closely mimics the conditions experienced by Warfighters exposed to blast resulting from an improvised explosive device. In this study, we evaluated the biochemical changes using in vivo longitudinal magnetic resonance spectroscopy (MRS) and behavioral profile following blast exposure.
Methods
Animal procedureFitch ferrets aged around postnatal day 100 were anesthetized with 4% isoflurane for 6 minutes and exposed to a BOP of ~140 KPa with 4-5msec positive pressure duration using an advanced blast simulator (ABS) that generates "free-field"-like blasts that closely resemble those experienced by Warfighters. The sham group underwent the anesthesia for the same duration without receiving the BOP exposure. Nine blast and eight sham ferrets were included in this study. MRS experiments were performed at 3-day, 7-day, 1-, 3- and 6-month post-BOP. Behavioral assessments were conducted at 1-, 3- and 6-month post-BOP.
Imaging experiments
MR experiments were performed on a Bruker Biospec 7T scanner (Bruker Biospin MRI GmbH, Germany). Anesthesia was maintained with about 3% isoflurane during the imaging session. Animal respiration rate was monitored, and body temperature was maintained at 37-38.5°C (SA Instruments, Inc., New York, USA). 1H-MRS data were obtained from a voxel (3.5 × 3.5 × 3.5 mm3) that covered the medial prefrontal cortex (mPFC) (Figure 1). A short-TE point-resolved spectroscopy pulse sequence (TR/ TE = 2,500/10 ms, NA = 360) was used for MRS data acquisition. The unsuppressed water signal from the prescribed voxel was used to determine the metabolite concentrations. 1H-MRS data were fitted using the LCModel (version 6.3-0G; LCModel Inc., Oakville, ON, Canada). A threshold of the Cramer-Rao lower bounds ≤35 % was used to select metabolite concentrations that had acceptable level of quantification reliability.
Behavioral assessment
An in-house trap test for ferrets was developed to measure anxiety and impulsivity behaviors (Figure 2A). The test used a modified skunk trap with a panel to control the opening and closing of the trap at one end. The animal was put inside the trap and allowed to acclimate for 2 min. After the acclimation, the panel was opened and the time that the animal took to vacate the trap (rear feet out) was recorded.
Data analysis
Repeated measures ANOVA was performed on MRS and behavioral measures with group as between subject effect and time post-blast as within subject effect. Interaction effect between group and time post-blast was also tested.
Results
A significant main effect of group was detected for the time the animals spent in the trap (p=0.01). The blast ferrets spent less time to exit the trap than the sham ferrets at 1- and 3-month post-blast (p=0.012 and p=0.005 respectively). The difference did not reach statistical significance at 6-month post-blast (Figure 2B). MRS data showed a significant group main effect for glutamate (p=0.047) and N-acetylaspartate (p=0.048) in the mPFC. Both metabolites increased significantly in the blast ferrets. Glutamate increased transiently at 1-month post-blast (p=0.024) while N-acetylaspartate remained high at both 1- and 3-month post-blast (p=0.039 and p=0.047 respectively) in the blast ferrets. In addition, significant effect of time post-blast was observed on taurine (p<0.001) and total creatine (p=0.026) which reflected developmental effects. Taurine was significantly lower at 3- and 6-month comparing to 3-day post-blast (p=0.006 and p<0.001 respectively) in both groups. Total creatine significantly increased in both groups from 7-day to 1-month post-blast (p=0.028) (Figure 3).Discussion and conclusion
In the trap test, the blast ferrets spent less time observing the surrounding environment than the sham ferrets before exiting the opening. This finding reveals increased impulsive decision making, as observed in previous rodent studies, which can be associated with prefrontal cortex-amygdalar axis dysfunction following TBI.Our finding of transiently increased glutamate is consistent with earlier reports during subacute stages following TBI, which were associated with cognitive dysfunction such as learning and memory problems 1. Increased levels of glutamate in prefrontal cortex were also observed in schizophrenia and mood disorders 2,3. N-acetylaspartate plays an important role in lipid synthesis and energy metabolism 4. Abnormally high brain N-acetylaspartate levels correlated with deficient axonal myelin sheath development 5. The N-acetylaspartate level in TBI ferrets showed significantly higher levels compared to sham animals at both 1- and 3-months, which may indicate a disrupted function of N-acetylaspartate in lipid synthesis or/and energy metabolism at the time. Considering that TBI is a risk factor of schizophrenia 6 and psychotic disorders 7, our MRS results, concomitant with the observed increased impulsivity, suggest a dysfunction of prefrontal cortex and potential secondary psychiatric disorders following BOP exposure.
Acknowledgements
This study was supported by Military Operational Medicine Research Program, Medical Research and Development Command (MRDC). The authors declare no conflict of interest.
References
1. Guerriero RM, Giza CC, Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep. 2015 May;15(5):27.
2. Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007 Dec 1;62(11):1310-6.
3. van Elst LT, Valerius G, Büchert M, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005 Nov 1;58(9):724-30.
4. Ariyannur PS, Moffett JR, Manickam P, et al. Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: implications for specialized acetyl coenzyme A metabolism in the CNS. Brain Res. 2010,1335, 1–13.
5. Madhavarao CN, Arun P, Moffett JR, et al. Defective N-acetylaspartate catabolism reduces brain acetate levels and myelin lipid synthesis in Canavan's disease. P Natl Acad Sci. 2005. 102, 5221–5226.
6. Molloy C, Conroy RM, Cotter DR, et al. Is traumatic brain injury a risk factor for schizophrenia? A meta-analysis of case-controlled population-based studies. Schizophr Bull. 2011 Nov;37(6):1104-10.
7. Fujii D, Fujii DC. Psychotic disorder due to traumatic brain injury: analysis of case studies in the literature. J Neuropsychiatry Clin Neurosci. 2012 Summer;24(3):278-89.
Figures
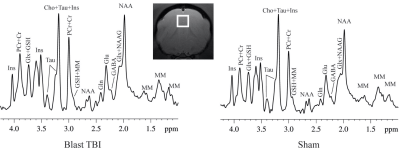
Figure 1. A demonstration of the MRS voxel in prefrontal cortex and spectrums of a blast and sham ferret at 3-day post-blast.
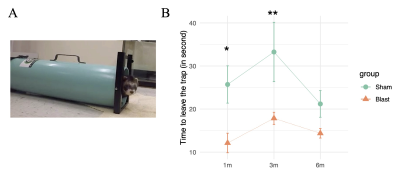
Figure 2. (A) The in-house trap test for ferret. (B) The time required to walk out from the trap of the sham and blast exposed ferrets at 1-, 3- and 6-month post-blast. *p<0.05, **p<0.01, differences between sham and blast animals.
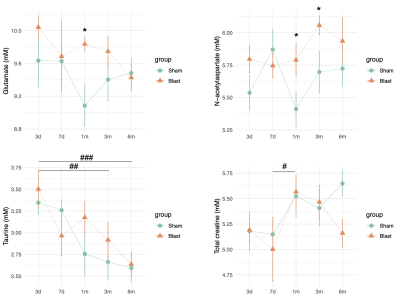
Figure 3. Brain metabolites levels in prefrontal cortex in sham and blast exposed ferret from 3-day to 6-month post-blast. *p<0.05, differences between sham and blast animals; #p<0.05, ##p<0.01, ###p<0.001, differences between visits.