3268
MRI assessment of placental oxygenation changes in response to maternal hyperoxygenation in a sheep model of human pregnancy1School of Biomedical Engineering & Imaging Sciences, King's College London, London, United Kingdom, 2Department of Medical Physics & Biomedical Engineering, University College London, London, United Kingdom, 3Early Origins of Adult Health Research Group, University of South Australia, Adelaide, Australia, 4Preclinical Imaging and Research Laboratories, South Australian Health and Medical Research Institute, Adelaide, Australia, 5Institute for Women's Health, University College London, London, United Kingdom, 6NIHR Biomedical Research Center, University College London Hospitals, London, United Kingdom
Synopsis
Abnormalities of placenta development and function may result in fetal growth restriction (FGR). Advances in MRI technology enable estimation of quantitative indices that reflect tissue diffusivity and oxygenation. We investigated the physiological impact of maternal hyperoxygenation on the placenta pregnant sheep. We applied a multi-compartment MRI signal model to measure the changes in oxygenation caused by changes in maternal blood oxygen level. The expected increase in feto-placental blood relaxation and feto-placental oxygen saturation with maternal hyperoxygenation was observed. Results suggested that diffusion and relaxation-based MRI is sensitive to acute changes in maternal and feto-placental oxygen level.
Introduction
Placental dysfunction is associated with fetal growth restriction (FGR) and is linked to poor exchange of oxygen and nutrients between the maternal and fetal circulations1. Several studies have reported an increase in human fetal oxygenation during 100% maternal oxygen inhalation2-4. In clinical obstetrics, a non-invasive method to estimate feto-placenta oxygenation is not available and current methods such as Doppler ultrasound measurements of umbilical blood flow estimate fetal well-being rather than directly measuring placental function.MRI methods such as Diffusion-Weighted imaging (DWI) are useful for providing non-invasive information on placental microstructure and function5,6. T2-relaxometry is a quantitative method that is sensitive to fetal blood oxygenation7 via differences in magnetic susceptibility of the oxygenated and deoxygenated haemoglobin in blood. However, fetal motion limits the effectiveness of these relatively slow techniques for direct assessment of the fetus, thus placental scans can provide a robust alternative. Nevertheless, placental imaging is complicated by the close proximity of maternal and fetal blood within an imaging voxel. To address this problem, we applied a multi-compartment model based on a previously used signal model of human placenta perfusion to separate fetal and maternal signal contributions7.
Methods
MethodsThe study was approved by the Animal Ethics Committee of the South Australian Health and Medical Research Institute.
MRI-protocol
Ewes underwent for MRI scans on a 3T Siemens Skyra Scanner (Erlangen,Germany). During the MRI scan, pregnant ewes were ventilated firstly in a normoxic state (2L O2 + 4L Room Air; respiratory rate (RR) 17-18 bpm; tidal volume, 10ml/kg) and then in a hyperoxemic state (6L O2 (i.e. 100% O2); RR, 17-18 bpm; tidal volume 10ml/kg). During each oxygenation state, DWI was performed at 7 b‐values (b = 0, 10, 20, 30, 50, 70, 100, 200, 300, 500, 600 s.mm-2) and T2-relaxometry at 10 echo times $$$T_E$$$ = (81, 90, 96, 120, 150, 180, 210, 240, 270, 300 ms). Data also acquired at b‐value 50 s.mm-2 and 200 s.mm-2 for $$$T_E$$$ = (81, 90, 120, 150, 180, 210, 240 ms).
Model-Fitting
The sheep-specific signal model is of the form:
$$S({\bf b},{\bf{T_E}})= S_0 \left[ e^{{-\bf b}d^*} \Big( f e^{-{\bf {T_E}}R_2^{f_b}}+ v e^{-{\bf {T_E}}R_2^{m_b}}\Big) + (1-f-v)e^{{-\bf b}d-{\bf {T_E}} R_2^{ts}} \right], \quad (1)$$
where $$$S$$$ is the measured MR signal and $$$S_0$$$ is the signal with $$$b=0$$$. The five model parameters are the feto-placenta blood volume fraction $$$f$$$, trophoblast diffusivity $$$d$$$, pseudo-diffusivity $$$d^*$$$, feto-placental blood relaxation $$$R_2^{f_b}=1/T_2^{f_b}$$$ and maternal blood volume fraction $$$v$$$. We used literature-based values for maternal blood relaxation $$$R_2^{m_b}=(150ms)^{-1}$$$ and tissue relaxation $$$R_2^{ts}=(46ms)^{-1}$$$.
The dependence of feto-placental blood oxygen saturation (FO2) on the effective $$$T_2^{f_b}$$$ is characterised by the Luz-Meiboom model9.
MRI-analysis
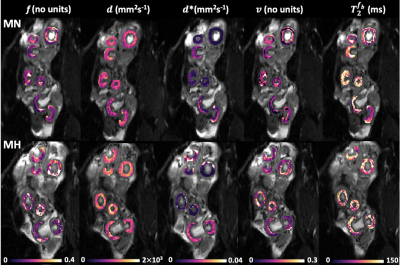
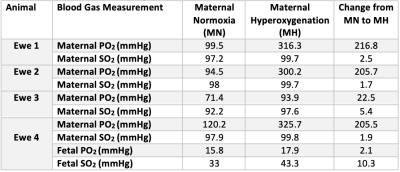
To minimise the effect of motion we used a nonrigid registration10 and manually delineated regions of interest containing the placentomes on the first b=0 image (ITK-SNAP Version 3.6.0, 2017). Voxel-wise fitting was performed with a Levenberg-Marquardt algorithm applied to Eq.(1) using in house software developed in MATLAB (MathWorks,Natick). Whilst 5 animals enrolled in the study, only 4 of these ewes had blood gas measurements. Blood gas values were collected from the maternal jugular vein of ewe 3, while for the other ewes they were collected from the maternal limb artery.
We calculated the difference of the mean maternal hyperoxia and the mean maternal normoxia MRI measurements for each ewe. One-sample t-test was then performed to examine the effect of maternal hyperoxygenation on the MRI parameters. Significance level was set to 5%.
Results
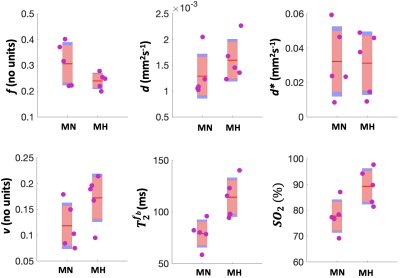
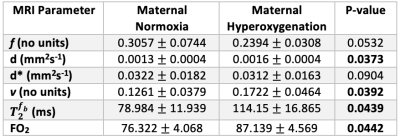
We observed a 43% increase in $$$T_2^{f_b}$$$ (P=0.0386) and 15% increase in FO2 (P=0.0311) with MH (Table 1). $$$f$$$ (P=0.0532) and $$$d^*$$$ did not change with MH but $$$v$$$ was significantly increased (P=0.0392). Whilst 5 ewes where enrolled in the study, only 4 of these ewes had blood gas measurements (Table 2). Maternal PO2 increased with MH by 168% from 96.40$$$\pm$$$20.03 to 259.03$$$\pm$$$110.59 (P=0.0401) and maternal SO2 increased by 3% from 96.3$$$\pm$$$2.77 to 99.2 $$$\pm$$$1.07 (P=0.0441).Discussion
Multi-compartment MRI is sensitive to changes in maternal and feto-placental oxygenation. Because of the model used, the changes in compartment volume fraction reflect a change in the placental oxygen gradient – increased oxygen in the deep placentome villi causes the estimated materno-placental volume fraction to increase as the oxygen gradient along the materno-placental villi is reduced. Comparably the estimated feto-placental volume decreases due to the increase in oxygenation of the feto-placental villi and the model association of high-T2 with the maternal blood compartment. This effect shows how the model assumptions are influenced by changes in oxygenation. The sheep placenta is also rate-limited by its mechanism of oxygen transfer and MRI can be used here to measure these non-linear effects in maternal to fetal oxygen exchange11. This is an important consideration for future translational studies adjusting for differences in sheep relative to human placentation. This study is limited by the small number of ewe sample and limited number of fetal samples.Conclusion
This study investigated sheep feto-placental oxygenation by the hyperoxic MRI in the pregnant sheep. Future work will enhance our modelling to further systematically understand the effect of changes in oxygenation on estimated by multi-compartment MRI parameters.Acknowledgements
This research was supported by the Wellcome Trust (210182/Z/18/Z, 101957/Z/13/Z, 203148/Z/16/Z), the EPSRC (NS/A000027/1) and an ARC Future Fellowship (Level 3; FT170100431) to JLM.References
1. Schneider H. Ontogenic changes in the nutritive function of the placenta. Placenta. 1996; 17(1):15–26.
2. Longo LD. Respiratory gas exchange in the placenta. Comprehensive Physiology 2011, Supplement 13: Handbook of Physiology, The Respiratory System, Gas Exchange: 351–401.
3. Khazin AF, Hon EH, Hehre FW. Effects of maternal hyperoxia on the fetus. Oxygen tension. Am J Obstet Gynecol 1971; 109(4): 628–637.
4. Young DC, Popat R, Luther ER, et al. Influence of maternal oxygen administration on the term fetus before labor. Am J Obstet Gynecol 1980; 136(3): 321–324.
5. Aughwane R, Mufti N, Flouri D et al. Magnetic resonance imaging measurement of placental perfusion and oxygen saturation in early-onset fetal growth restriction. BJOG: Int J Obstet GY. 2021; 128(2): 337–345.
6. Flouri D, Darby JR, Holman SL, Perumal SR, et al. Mangetic resonance imaging of placentome development in the pregnant ewe. Placental. 2021; 61–69.
7. Melbourne A, Aughwane R, Sokolska M, et al. Separating fetal and maternal placenta circulations using multiparametric MRI. Magn Reson Med. 2018; 00:1–12.
8. Danielson L, McMillen IC, Dyer JL, Morrison JL. Restriction of placental growth results in greater hypotensive response to alpha-adrenergic blockade in fetal sheep during late gestation. J Physiol. 2005; 563:611–620.
9. Wright GA, Hu BS, Macovski A. Estimating oxygen saturation of blood in vivo with MR imaging at 1.5T. J Magn Reson Imaging. 1991; 1: 275–283.
10. Flouri D, Owen D, Aughwane R, et al. Improved fetal blood oxygenation and placental estimated measurements of diffusion-weighted MRI using data-driven Bayesian modeling. Magn Reson Med. 2020; 83: 2160 – 2172.
11. Silver M, Comline RS. Transfer of gases and metabolites in the equine placenta: A comparison with other species. J Reprod Infertil. 1975; 23:589–594.
Figures