3265
What to do for an accurate cross-sectional and longitudinal processing of diffusion tensor imaging: application to frontotemporal dementia1Department of Medical Physics and Biomedical Engineering, University College London, London, United Kingdom, 2Dementia Research Centre, Department of Neurodegenerative Disease, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 3Centre for Medical Image Computing (CMIC), Department of Computer Science, University College London, London, United Kingdom
Synopsis
Introduction
To have reliable biomarkers capable of detecting true changes in white matter (WM) regions, image analyses require methodological steps to minimise the effects of image artefacts and to achieve good spatial correspondence. This is important for cross-sectional studies, but even more so for longitudinal studies, especially in patients with brain abnormalities such as in dementia.Here we considered a state-of-the-art approach for DTI analysis, using high quality images which were acquired on a state-of-the-art 3T MR scanner to maximise the accuracy of DTI metrics. We applied the improved method to a small cohort of FTD patients to verify if the results we obtained could replicate the findings in the existing literature.
Method and Materials
Participants: Twenty patients with FTD (behavioural variant FTD (bvFTD) = 10; non-fluent variant primary progressive aphasia (nfv-PPA) = 3, primary progressive aphasia not otherwise specified (PPA-NOS) = 4, semantic variant primary progressive aphasia (sv-PPA) = 4) and twenty healthy control individuals (HC) were selected to apply this method. Due to the small size of the sample, we merged the three language variants into the PPA group. To test the longitudinal methods, we used MRI data available from 32 of the participants (bvFTD=10, PPA=9, HC=13) at 12-month follow-up.MRI acquisition: Diffusion weighted imaging (DWI) were acquired on a 3T Siemens Prisma scanner, with the following imaging parameters: SE-EPI, TE/TR = 90/7300 ms, 96 × 96 acquisition matrix and 59 slices, 2.5 mm isotropic voxels, 64 directions at b=1000 s/mm2, 5 b=0 images. We used two repeated DWI acquisitions in this work1. The T1-weighted images were acquired with the following parameters: matrix 256 × 256, 208 slices, slice thickness = 1.1 mm, TE/TR = 2.93/2200 ms, TI = 850 ms, flip angle = 8°.
Imaging processing: Fig. 1 illustrates the processing steps. We used a subject-specific binary brain mask in the T1-space to constrain processing to the brain tissue and improve the registration performance. Motion artifacts were corrected by using an affine co-registration between the diffusion weighted images and average b=0 images. And we corrected the susceptibility induced geometric distortion using phase unwrapping approach2. Within-subject DTI templates were generated using an iterative process of rigid registration, followed by affine then nonlinear registration. A groupwise (GW) template was created from in the same way using DTI-TK (http://dti-tk.sourceforge.net)3.
Statistical analyses: Analysis of covariance adjusting for age and gender was used to compare DTI metrics across bvFTD, PPA, and HC groups for cross-sectional investigation. Moreover, we compared the annual change in each DTI metric considered, using the available two time-points for each participant, and determined the annual change in the measurement as follows (using FA as an example):
Annual change in FA = (FAsecond timepoint – FAfirst timepoint)/time interval (years)
Two sample t-tests were performed between disease group and HC cohort respectively. Two-tailed p < 0.05 were considered statistically significant.
Results
Applying the methods ensured that all steps worked by performing rigorous data control after each pre-processing step, and when the method was applied to the FTD cohort, we found significant results both in the cross-sectional and longitudinal analyses.The cross-sectional significant results between bvFTD and PPA versus HC groups are shown in Fig. 2 and Fig. 3. There was a differential pattern of WM tract changes in the two FTD groups.
Fig. 4 and Fig. 5 show the significant results for the longitudinal changes between bvFTD and PPA versus HC. Each FTD subtype had distinct patterns of longitudinal WM alteration. In bvFTD, some WM tracts (like cingulum gyrus and external capsule) did not show changes over time, whilst additional WM abnormalities (such as the left posterior thalamic radiation) were observed. PPA showed more extensive WM changes than bvFTD, with changes no longer limited to the left hemisphere as seen at baseline.
Discussion and Conclusion
Here we considered the different steps that are important to address when working with cross-sectional and longitudinal DTI data, especially in dementia. We found that it is important to include the best data acquisition and processing because this allows robust results.After applying the state-of-the-art methods on a small sample of FTD patients, we managed to replicate findings reported in the literature. We found changes in tracts already reported for bvFTD (corpus callosum, cingulum, fornix, corona radiata, external capsule) and PPA (left uncinate fasciculus, fornix)4-10. Moreover, we found additional WM tracts (left inferior cerebellar peduncle and left sagittal stratum, posterior internal capsule in bvFTD, left inferior cerebellar peduncle and bilateral anterior internal capsule in PPA) that showed longitudinal changes compared with some existing studies4, 5.
The current work not only used high-quality image acquired with the same protocol on the same scanner (cross-sectionally and longitudinally), but it also applied an improved DTI processing pipeline with the most advanced tools that correct for the typical issues of DTI (motion, eddy current and susceptibility-induced distortion). Moreover, we performed an improved spatial normalization by creating a GW template which is unbiased towards any single timepoint3.
The results indicated that when addressing the technical issues in DTI, we could replicate the typical WM features seen in FTD even in small cohorts. This underlies the importance of applying advanced processing technique when running analyses in clinical populations.
Acknowledgements
We thank the research participants for their contribution to the study. The Dementia Research Centre is supported by Alzheimer's Research UK, Alzheimer's Society, Brain Research UK, and The Wolfson Foundation. This work was supported by the National Institute for Health Research (NIHR) Queen Square Dementia Biomedical Research Unit and the University College London Hospitals Biomedical Research Centre, the Leonard Wolfson Experimental Neurology Centre (LWENC) Clinical Research Facility, and the UK Dementia Research Institute, which receives its funding from UK DRI Ltd, funded by the UK Medical Research Council, Alzheimer's Society and Alzheimer's Research UK. MB’s work was also supported by the UK Dementia Research Institute which receives its funding from DRI Ltd, funded by the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK. JDR is supported by the Miriam Marks Brain Research UK Senior Fellowship and has received funding from an MRC Clinician Scientist Fellowship (MR/M008525/1) and the NIHR Rare Disease Translational Research Collaboration (BRC149/NS/MH).
References
1. Farrell, J.A., et al., Effects of signal‐to‐noise ratio on the accuracy and reproducibility of diffusion tensor imaging–derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5 T. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine, 2007. 26(3): p. 756-767.
2. Daga, P., et al., Susceptibility artefact correction using dynamic graph cuts: application to neurosurgery. Medical image analysis, 2014. 18(7): p. 1132-1142.
3. Keihaninejad, S., et al., An unbiased longitudinal analysis framework for tracking white matter changes using diffusion tensor imaging with application to Alzheimer's disease. Neuroimage, 2013. 72: p. 153-163.
4. Mahoney, C.J., et al., Longitudinal diffusion tensor imaging in frontotemporal dementia. Annals of neurology, 2015. 77(1): p. 33-46.
5. Lam, B.Y., et al., Longitudinal white matter changes in frontotemporal dementia subtypes. Human brain mapping, 2014. 35(7): p. 3547-3557.
6. Gordon, E., J.D. Rohrer, and N.C. Fox, Advances in neuroimaging in frontotemporal dementia. Journal of Neurochemistry, 2016. 138: p. 193-210.
7. Elahi, F.M., et al., Longitudinal white matter change in frontotemporal dementia subtypes and sporadic late onset Alzheimer's disease. NeuroImage: Clinical, 2017. 16: p. 595-603.
8. Agosta, F., et al., White matter damage in frontotemporal lobar degeneration spectrum. Cerebral cortex, 2012. 22(12): p. 2705-2714.
9. Mahoney, C.J., et al., Profiles of white matter tract pathology in frontotemporal dementia. Human brain mapping, 2014. 35(8): p. 4163-4179.
10. Mahoney, C.J., et al., White matter tract signatures of the progressive aphasias. Neurobiology of aging, 2013. 34(6): p. 1687-1699.
Figures
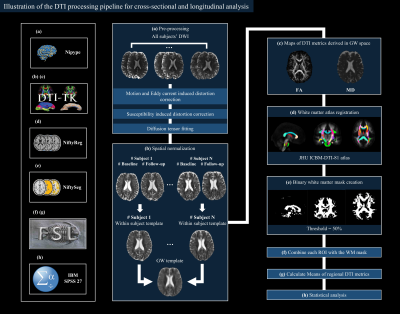
Figure 1. Illustration of the diffusion tensor imaging processing pipeline for both cross-sectional and longitudinal analyses. The left column shows the tools used in each step.

Figure 2. Significant regions in the bvFTD group from the cross-sectional investigation compared with healthy control groups. Regions that were significantly altered in the bvFTD group are shown in the glass brain representations. The boxplots for FA and MD values in the regions showing significant results are around the glass brain.

Figure 3. Significant regions in the PPA group from the cross-sectional investigation compared with healthy control groups. Regions that were significantly altered in the PPA group are shown in the glass brain representations. The boxplots for FA and MD values in the regions showing significant results are around the glass brain.

Figure 4. Significant regions in the bvFTD group from the longitudinal investigation compared with healthy control groups. Regions that showed statistically significant annual change in DTI metrics in the bvFTD group are shown in the glass brain representations. The boxplots for annual change in FA and MD in the regions showing significant results are around the glass brain.
