3259
Hippocampal subfield-specific alterations in post-stroke dementia with subcortical lesion1Key Laboratory for Biomedical Engineering of Ministry of Education, Department of Biomedical Engineering, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, Hangzhou, China, 2College of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou, China, Hangzhou, China, 3Department of Neurology, Neuroscience Center, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, China,, Hangzhou, China, 4Department of Radiology, Neuroscience Center, Sir Run Run Shaw Hospital, Zhejiang University, Hangzhou, China, Hangzhou, China
Synopsis
The present study aimed to explore functional and structural alterations in hippocampus and its subfields in post-stroke dementia (PSD). We collected resting-state fMRI and DTI data from 24 PSD patients, 36 post-stroke nondemented (PSND) patients and 21 normal controls (NC). Comparisons between groups found that PSD patients showed subfield-specific changes in the microstructures of hippocampus compared with the other two groups. Moreover, we not only found more PSD-related changes of functional connectivity with cortical regions in the subfields than entire hippocampus, but also revealed the different contributions of subfields on the connectivity changes of entire hippocampus.
Introduction
The hippocampus consists of several subfields with different functions and anatomical connections, which have been reported to show a selective atrophy in dementia diseases [1,2]. Our recent study revealed dementia-specific atrophy in the hippocampal subfields in post-stroke dementia (PSD) [3]. However, it remains unclear how functional and structural connectivity between hippocampal subfields and cortical regions would be affected by PSD.Methods
This is a cross-sectional study. 24 PSD, 36 post-stroke nondemented (PSND) patients, and 21 NCs matched in age were recruited in the present study. The lesions in stroke patients all located on the subcortical cortex. Mini-Mental State Examination (MMSE) and miniCog were used to assess the cognitive function. We collected the multi-modal MRI data from T1-weighted imaging, resting-state fMRI (rs-fMRI), and diffusion tensor imaging (DTI). After the preprocessing for rs-fMRI and DTI images, we first calculated the fractional amplitude of low-frequency fluctuations (fALFF), fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD) and radial diffusivity (RD) maps for each participant. Then, we used FreeSurfer 6.0 to segment the T1-weighted image and obtained hippocampal subfields at the individual level, which were further registered to fMRI and DTI spaces using a FLIRT method [4,5] and extracted the averaged values of fALFF/FA/MD/AD/RD in the entire hippocampus and its subfields in each participant. Finally, we registered the AAL template from MNI to native space, and constructed the functional and structural connectivity between cortical regions and hippocampus as well as its subfields for each participant.In statistical analysis, we used a linear mixed model with age, sex, education, head motion and lesion information as the covariates and with subject factor as a random variable, to compare the difference between the groups and evaluate the relationship between MRI measurements and clinical assessments.
Results
PSD patients showed significant lower MMSE and miniCog scores compared with PSND patients, indicating that their cognitive functions were impaired. MRI analysis found that compared with NC, PSD showed significant increases in diffusivity in the left CA1 and tail (FDR correction, p < 0.05). Relative to PSND, PSD showed a significant decrease in diffusion anisotropy in the right CA3 and a significant increase in diffusivity in the right tail (FDR correction, p < 0.05) (Figure 1). Also, most subfields displayed a trend of decrease in the diffusion anisotropy and increase in the diffusivity, from NC to PSND to PSD. Moreover, we found significantly positive correlations between the diffusivity in bilateral hippocampal tails and the duration of illness in the PSND but not in the PSD groups (FDR correction, p < 0.05) (Figure 2). Importantly, we found PSD-related changes in functional connectivity with the cortical regions (temporal and occipital cortexes) in the subfields, which not only almost replicated the findings in the entire hippocampus, but also revealed several new changes associated with the dementia (FDR correction, p < 0.05) (Figure 3). Interestingly, different subfields showed distinct contributions on the changes of functional connectivity of entire hippocampus (Figure 4). We did not find significant differences among three groups in the fALFF and structural connectivity between cortical regions and hippocampus as well as its subfields.Discussion
In this study, we focused on the function and structure of hippocampal subfields, and found that PSD patients showed abnormal diffusions (e.g., decrease in fractional anisotropy and increase in diffusivity) in several hippocampal subfields but not in the entire hippocampus. This indicates that PSD may have a subfield-specific effect on the hippocampal microstructure, which is consistent with the previous reports [3,6]. We also found that, as the disease progresses, the diffusivity in the hippocampal tails showed a significant increase in PSND patients, which might be associated with hippocampal microvasculature changes upon stroke [7]. Moreover, we found more PSD-related changes of functional connectivity in the subfields than entire hippocampus, and the subfields presented different contributions on the connectivity changes of entire hippocampus. We speculate that the microstructural damages in the hippocampal subfields may affect the functional connectivity between hippocampus and cortical regions.Conclusion
We found that PSD patients showed microstructure alterations and abnormal functional connectivity associated with cortical regions in the hippocampal subfields. The diffusivity changes in hippocampal tails could predict the progression of disease in patients after stroke. These findings suggest PSD has a significant effect on hippocampal subfields, and the subfield analysis provides a new insight toward the underlying mechanism of PSD.Acknowledgements
This work is supported by the Youth Program of National Natural Science Foundation of China (82001907), Natural Science Foundation of Zhejiang Province of China (LY19H090027), and China Postdoctoral Science Foundation (2020M671726).
References
1. Jack CR, Petersen RC, Xu Y, et al. Rates of Hippocampal Atrophy Correlate with Change in Clinical Status in Aging and AD. Neurology. 2000;55:484-490.
2. van de Pol LA, Hensel A, van der Flier WM, et al. Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(4):439-442.
3. Zhao Z, Cai H, Zheng W, et al. Atrophic Pattern of hippocampal subfields in post-stroke demented patient. Journal of Alzheimer’s Disease. 2021;80(3):1299-1309.
4. Rutland JW, Brown S, Verma G, et al. Hippocampal subfield-specific connectivity findings in major depressive disorder: A 7 Tesla diffusion MRI study. Journal of Psychiatric Research. 2019;111:186-192.
5. Smagula SF, Karim HT, Rangarajan A, et al. Association of Hippocampal Substructure Resting-State Functional Connectivity with Memory Performance in Older Adults. American Journal of Geriatric Psychiatry. 2018;26(6):690-699.
6. Zhao Z, Cai H, Huang M, et al. Altered Functional Connectivity of Hippocampal Subfields in Poststroke Dementia. Journal of Magnetic Resonance Imaging. 2021;54(4):1337-1348.
7. Burke MJC, Nelson L, Slade JY, Oakley AE, Khundakar AA, Kalaria RN. Morphometry of the hippocampal microvasculature in post-stroke and age-related dementias. Neuropathology and Applied Neurobiology. 2014;40(3):284-295.
Figures
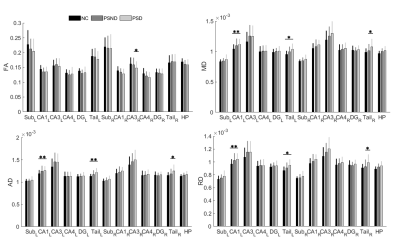
Figure 1: The differences of diffusion measurements in the entire hippocampus and its subfields between the groups. PSD showed subfield-specific decreases in diffusion anisotropy in the right CA3 and increases in diffusivity in the left CA1 and bilateral hippocampal tails (FDR correction, p < 0.05). FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity; Sub: subiculum; CA: cornu ammonis; DG: dentate gyrus; HP: entire hippocampus; L: left; R: right. *p < 0.05 and **p < 0.01. The error bars represent the standard deviation.
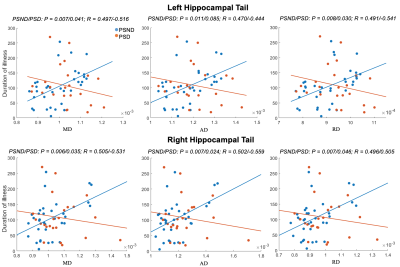
Figure 2: Correlations between the diffusivity in bilateral hippocampal tails and the duration of illness in the two patient subgroups. PSND showed significant positive correlations (FDR correction, p < 0.05), whereas PSD showed insignificant correlations (FDR correction, p > 0.05). In each subplot, red/blue dots represent the PSD/PSND patients. The unit of duration of illness is day.
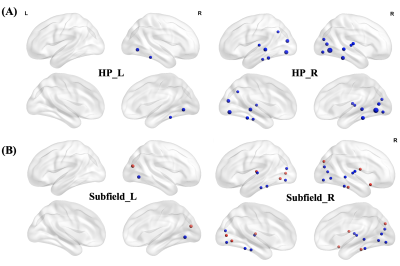
Figure 3: The differences between NC and PSD groups in functional connectivity. (A) PSD showed significantly reduced functional connectivity between entire HP and temporo-occipital cortex (blue spheres) compared with NC group. The size of sphere represents the degree of difference between groups (FDR correction, p < 0.05). (B) The subfield analysis revealed more PSD-related changes in functional connectivity with the cortical regions (FDR correction, p < 0.05), which not only replicated the findings (blue spheres) of entire HP but also found new changes (red spheres).
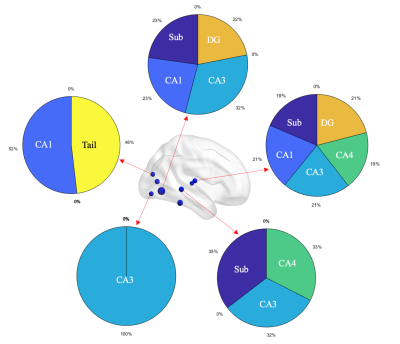
Figure 4: A schematic diagram showing the contributions of subfields on functional connectivity of entire hippocampus. Different subfields had distinct contributions on the changes of functional connectivity with cortical regions in the entire HP. For example, several connectivities were associated with only one subfield while other connectivities were correlated with multiple subfields. Blue spheres represent the cortical regions showing between-group differences in the functional connectivity with entire HP. The pie charts represent the contributions of subfields.