3257
Brain structure, amyloid and behavioural features for predicting clinical progression in subjective cognitive decline1National University of Singapore, Singapore, Singapore, 2Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Synopsis
Subjective cognitive decline (SCD) is a risk factor for dementia. However, multiple pathologies, including amyloid deposition, cerebrovascular pathology, and depression, contribute to the heterogeneity in SCD. We included 170 non-demented elderly with 2-years follow-up to examine brain structural abnormalities in SCD. We found progressive and stable SCD individuals differed in deep white matter hyperintensities and temporoparietal grey matter atrophy. We used multi-model brain and behavioural factors to predict cognitive impairment and dementia progression. Periventricular white matter hyperintensities mediated the effect of amyloid accumulation on cognitive decline and disease severity, while depressive symptoms directly predicted disease severity.
INTRODUCTION
As a potential preclinical Alzheimer’s dementia marker, subjective cognitive decline (SCD) reveals a higher risk of future cognitive decline and conversion to dementia1–3. However, dementia-related clinical progression could result from multiple pathologies, including amyloid deposition4,5, cerebrovascular disease (CeVD)6,7, and psychiatric disorders8–10. Potentially, this could contribute to the considerable heterogeneity within the SCD population, resulting in the uncertainty of SCD clinical trajectories11. Given the multiple pathways of the clinical progression, it is crucial to incorporate multi-modal information into one longitudinal study to understand the relationship between different pathologies. By assessing structural MRI, amyloid PET, and psychiatric symptoms, we aimed to (1) investigate the changes in neurodegeneration and CeVD burden in SCD over time and their relationship with disease progression; and (2) predict cognitive impairment and dementia progression based on multi-model brain and behavioural factors. Specifically, we hypothesised that progressive SCD individuals had more WMH burden and grey matter atrophy increase over time. We also expected risk factors predict WMH burden and grey matter atrophy at baseline, which in turn forecast increases of WMH burden and grey matter atrophy two years later. Depending on the underlying pathologies, brain structural changes may mediate the impacts of risk factors on cognitive function and disease severity.METHODS
We identified 170 non-demented elderly with 2-years follow-up, including 101 cognitively normal controls (NC) and 69 SCD individuals12. Based on the clinical progression during the 2-year follow-up, we divided the SCD participants into 54 stable-SCD (S-SCD) and 15 progressive SCD (P-SCD) individuals (who developed cognitive impairment). We computed white matter hyperintensities (WMH) volumes from FLAIR images13,14 and voxel-wise grey matter volumes from T1-weighted images15.To understand the relationship between SCD and CeVD (Aim 1), we compared these imaging metrics among the participant groups at the baseline and their changes two years later. To address aim 2, we constructed a path model using the structural equation modelling method to predict future cognitive impairment and dementia progression. The predictors included amyloid deposition, age, and depressive symptoms. We considered WMH burden and grey matter atrophy at baseline and changes at year two as mediators. Lastly, we included cognitive function assessed by the Mini-Mental State Exam (MMSE)16 and disease severity estimated by the Clinical Dementia Rating (CDR) Scale17 as outcomes in the model.
RESULTS
We found increased amyloid deposition and severer depression in SCD at baseline. SCD showed higher periventricular WMH at baseline and faster progression in the follow-up than NC. However, only P-SCD had higher deep WMH at baseline and faster progression in the follow-up than S-SCD and NC. In addition, P-SCD exhibited faster grey matter volume decline over two years in the medial temporal gyrus and precuneus than S-SCD and NC.Path analyses showed that individuals with higher baseline age and amyloid were linked to higher baseline periventricular and deep WMH, which predicted WMH increase in two years. Periventricular WMH mediated the effects of baseline age and amyloid deposition on MMSE increase. Periventricular WMH also mediated the effect of amyloid deposition on CDR increase. In addition, baseline depressive symptoms had a direct effect on CDR increase. For grey matter changes, baseline age significantly predicted precuneus atrophy changes over two years. Periventricular WMH was significantly related to baseline age and precuneus atrophy changes over time but did not mediate the effects of baseline age on precuneus atrophy changes. Medial temporal gyrus atrophy change was significantly related to baseline amyloid deposition and CDR increase over time but did not mediate the effects of baseline amyloid deposition on CDR increase.
DISCUSSION
Our analyses revealed that, while periventricular WMH burden was higher in SCD individuals than controls, progressive SCD individuals but not stable SCD individuals had more deep WMH burden at baseline and faster deep WMH accumulation over the 2-year follow-up. Progressive SCD had also accelerated temporoparietal grey matter atrophy.Taking advantage of multi-modal imaging study design, we also showed distinct pathways contributing to disease progression. Periventricular WMH burden mediated the impact of amyloid deposition on cognitive functions and disease severity. Depressive symptoms predict disease severity, dissociating the psychiatric pathology from the amyloid and CeVD pathologies.
CONCLUSIONS
Our work highlights the importance of multi-modal imaging methods and longitudinal designs in understanding the heterogeneity of SCD trajectories and the distinct pathways of multiple clinical pathologies. Our results have implications in patient stratification and early intervention of SCD.Acknowledgements
This research was supported by the Biomedical Research Council, Singapore (BMRC 04/1/36/372), the National Medical Research Council, Singapore (NMRC/CBRG/0088/2015, NMRC/CIRG/1390/2014, and NMRC/CIRG/1446/2016), and Duke-NUS Medical School Signature Research Program funded by Ministry of Health, Singapore. Besides, this study was funded by the National Natural Science Foundation of China (Grant No. 81901707). Finally, we gratefully acknowledge financial support from the China Scholarship Council (CSC).The ADNI funded this project's data collection and sharing (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012).
References
1 Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 2014; 10: 844–852.
2 Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer’s disease. Annu Rev Clin Psychol 2017; 13: 369–396.
3 Ronnlund M, Sundstrom A, Adolfsson R, Nilsson LG. Subjective memory impairment in older adults predicts future dementia independent of baseline memory performance: Evidence from the Betula prospective cohort study. Alzheimers Dement 2015; 11: 1385–92.
4 Samieri C, Proust-Lima C, Glymour MM, Okereke OI, Amariglio RE, Sperling RA et al. Subjective cognitive concerns, episodic memory and the APOE ε4 allele. Alzheimers Dement 2014; 10: 752-759.e1.
5 Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 2014; 83: 1359–65.
6 Hilal S, Ikram MK, Saini M, Tan CS, Catindig JA, Dong YH et al. Prevalence of cognitive impairment in Chinese: Epidemiology of Dementia in Singapore study. J Neurol Neurosurg Psychiatry 2013; 84: 686–92.
7 Hilal S, Liu S, Wong TY, Vrooman H, Cheng C-Y, Venketasubramanian N et al. White matter network damage mediates association between cerebrovascular disease and cognition. J Cereb Blood Flow Metab 2021; : 0271678X21990980.
8 da Silva J, Gonçalves-Pereira M, Xavier M, Mukaetova-Ladinska EB. Affective disorders and risk of developing dementia: systematic review. Br J Psychiatry 2013; 202: 177–186.
9 Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; 202: 329–335.
10 Steffens DC, Potter GG. Geriatric depression and cognitive impairment. Psychological Medicine 2008; 38: 163–175.
11 Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. International Psychogeriatrics 2008; 20: 1–16.
12 ADNI | Alzheimer’s Disease Neuroimaging Initiative. http://adni.loni.usc.edu/ (accessed 9 Nov2021).
13 Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage 2012; 59: 3774–3783.
14 Seo SW, Lee J-M, Im K, Park J-S, Kim S-H, Kim ST et al. Cortical thinning related to periventricular and deep white matter hyperintensities. Neurobiology of Aging 2012; 33: 1156-1167.e1.
15 Gaser C, Dahnke R, Kurth K, Luders E, Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Disease Neuroimaging Initiative. A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. NeuroImage In review.
16 Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198.
17 Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412–2414.
Figures
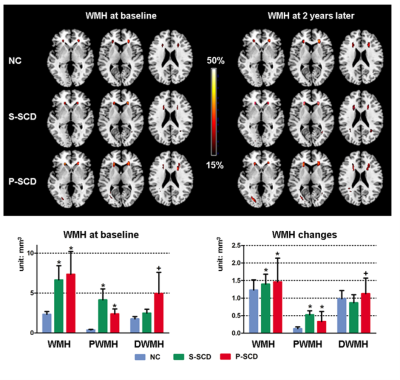
Figure 1. Progressive SCD had higher deep WMH at baseline and faster volume increase over the two-year follow-up. Only areas with WMH appearing in at least 15% of the participants within each group were shown. Top: colours represent the proportion of participants showing WMH in the voxel. Bottom: reported at p < 0.05, Bonferroni corrected, unit: mm3. Blue, green and red bar represents normal controls (NC), Stable SCD (S-SCD) and P-SCD, respectively. ‘*’ represents significant differences compared to NC; ‘+’ represents significant differences compared to both the S-SCD and NC.
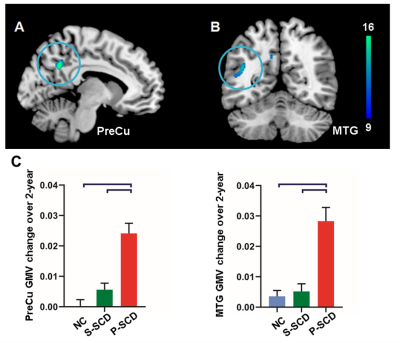
Figure 2. Accelerated temporoparietal grey matter atrophy in progressive SCD individuals. Accelerated atrophy of (A) left precuneus (PreCu) and (B) left middle temporal gyrus (MTG), controlling for age, gender and education (corrected by Gaussian random field, p<0.001 at the height level, p<0.05 at the cluster level). Note: square brackets represent a significant difference of progressive SCD (P-SCD) compared to both stable SCD (S-SCD) and controls (NC), p<0.001.
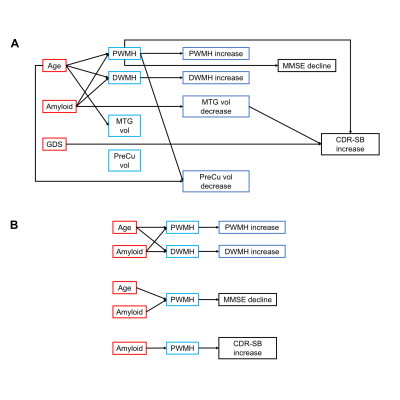
Figure 3. Schematic diagrams of the path model. A. A diagram of the path model including predictors, mediators and outcomes. B. Highlighting the significant mediations in the path models. The arrows indicate significant relationships between the two variables. Education, APOE ε4 and sex are covariates. Abbreviations: PWMH/DWMH, periventricular/deep white matter hyperintensities; SCD, subjective cognitive decline; GMV, grey matter volume; CDR-SB, clinical dementia rating sum of boxes.