3243
Deuterium metabolism imaging of rat brain at 9.4T using a double-nuclear transceiver
Feng Du1,2, Jiawen Yuan1,3, Nan Li1,2, Chao Zhou1,2, Qian Wan1,2, Qikai Qin4, Garth J. Thompson4, Qiong Ye5, Xiaoliang Zhang6, Xin Liu1,2, Hairong Zheng1,2, and Ye Li1,2
1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province, Shenzhen, China, 3Southern University of Science and Technology, Shenzhen, China, 4iHuman Institute, ShanghaiTech University, Shanghai, China, 5High Magnetic Field Laboratory, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei, China, 6Department of Biomedical Engineering, State University of New York, Buffalo, NY, United States
1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province, Shenzhen, China, 3Southern University of Science and Technology, Shenzhen, China, 4iHuman Institute, ShanghaiTech University, Shanghai, China, 5High Magnetic Field Laboratory, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei, China, 6Department of Biomedical Engineering, State University of New York, Buffalo, NY, United States
Synopsis
Deuterium metabolic imaging (DMI) is an emerging technique, which can measure the metabolism in the brain non-invasively after intake of deuterated glucose. In this work, we construct a versatile and dedicated 1H/2H dual-nuclear transceiver for a 9.4T pre-clinical research system. The performance of the transceiver was evaluated by measuring the in vivo 2H spectra data. The metabolites of water, glucose, Glx and lactate after glucose infusion were quantified in healthy and glioma rats using the constructed double-nuclear transceiver.
Introduction
Deuterium Metabolic Imaging (DMI) is a molecular metabolic imaging technology that has emerged in recent years. DMI enables the investigation of metabolic pathways by tracking deuterated substrates and their downstream metabolites. It provides a new tool for the study of tumor and neurodegenerative diseases 1-3. As the sensitivity of weak nuclides scale supralinear with the magnetic field strength, the application of 2H imaging at ultra-high field leads to a significant gain in signal to noise ratio (SNR), which can be employed to reduce scan time or to improve spatial resolution4-5. In this work, a versatile and dedicated 1H/2H double-nuclear transceiver for signal excitation and reception of DMI on 9.4T pre-clinical research system was constructed and tested. The S-parameters was measured in workbench to analyze the performance of the double-nuclear transceiver. The natural abundance of deuterium signal before glucose infusion in rat brain was acquired to evaluate the stability of performance. 2H spectra data were acquired at healthy and glioma rat brain after glucose infusion using the constructed double-nuclear transceiver to verify the ability of high sensitivity acquisition of the signals.Method
The double-nuclear transceiver employed in this work consisted of 2 looped coils forming the double layer, as shown in Fig. 1. The outer loop of the double layer coil was set to 25 * 25 mm and tuned to operate at the 1H resonance frequency of 400.3 MHz. The inner loop, for 2H, was 5mm smaller than the 1H coil, with a dimension of 20 * 20 mm. It was tuned to operate at the resonance frequency of 61.4 MHz for 2H. This independent-loop design was versatile, as it enabled the optimization of the sensitivity and RF field distributions of the two coils separately. The outer loop elements were matched to 50 Ohm and tuned to the target frequencies with non-magnetic fixed capacitors (Series 100B, American Technical Ceramics, USA) and two high voltage non-magnetic variable capacitors (NMAJ25HV, 1~23 pF, Voltronics, USA) were employed to connect in series in each circuit for matching and frequency adjustment. For the inner loop, tuning and matching was achieved by two high-voltage non-magnetic variable capacitors, which can reduce the deterioration of RF circuit performance caused by the parasitic parameters of discrete devices at the transmitting frequency. Bench measurements were performed in a Network Analyzer (Agilent Technologies, Palo Alto, USA). The reflection (S11) and transmission (S21) coefficient were measured for both nuclei to evaluate the coil tuning, impedance matching conditions and the decoupling between the two nuclides. The data was acquired at a Bruker BioSpec 94/30 9.4T animal system (Bruker, Ettlingen, Germany) with the constructed double-nuclear transceiver placed over the rat head. The animal was anesthetized with isoflurane during the MR data acquisition. The localized shimming was performed on rat brain using the 1H coil of double-nuclear transceiver. T2 weighted image (T2WI) was acquired as follows: TR/TE=3000/81 ms, matrix = 160 * 120, resolution = 0.2mm * 0.2mm. The baseline 2H spectra used to quantify the natural abundance of 2H was acquired before the infusion of deuterated glucose. Single pulse sequence was used for 2H spectra. The scan parameters were: TR = 300ms, FA = 60°, bandwidth = 2kHz, resolution = 256, averages = 600, acquisition time=3 min. Subsequently, a 2-minute tail vein infusion of custom-synthesized [2,3,4,6,6’-2H5] glucose (1.95 g/kg body weight) was administered. The 2H spectra were required for 50 repetitions, lasting a total time of 150 minutes after the glucose infusion with the same scanning protocol. The 2H spectra were analyzed by TopSpin (Bruker, version 4.1.3, USA).RESULT
The measured S-parameters of the proposed double-nuclear transceiver were shown in Table 1. The reflection coefficients (S11 and S22) were both less than -15 dB at 400.3MHz (1H) and 61.4MHz (2H). The transmission coefficient (S21) is less than -20 dB at low frequency and -40 dB at high frequency, which indicates a good decoupling between the two nuclides. The intensity values of the natural abundance of 2H in six rat brains acquired by the constructed double-nuclear transceiver was shown in Table 2. The coefficient of variation of 0.02 for the intensity values implies the stable performance of the transceiver. Figures 2 and 3 present the 2H spectra and the metabolites in a healthy rat brain and one rat with glioma after deuterated glucose injection using the constructed double-nuclear, respectively.Conclusion
In this work, we designed and constructed a versatile and dedicated 1H/2H dual-nuclear transceiver for DMI on 9.4T pre-clinical research system. The 2H spectra data in healthy and glioma rats verified the ability of the high SNR deuterium MRS measurements. Potentially, the sensitivity could be further increased by optimizing the size of the transceiver. In the future, more advanced acquisition strategies will be investigated to reduce the acquisition time or to increase the spatial resolution for the non-invasive metabolic imaging.Acknowledgements
This work was supported in part by the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB25000000); National Key R&D Program of China, 2021YFE0204400; city grant RCYX20200714114735123.References
1. De, Feyter Henk M., et al. "Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo." Science Advances 4.8(2018):eaat7314. 2. Straathof, M., et al. "Deuterium Metabolic Imaging of the Healthy and Diseased Brain." Neuroscience 9(2021). 3. Warburg, O., F. Wind, and E. I. Negelein. "The Metabolism of Tumors in the Body." The Journal of General Physiology 8.6(1927):519-530. 4. de, Graaf Robin A., et al. " On the magnetic field dependence of deuterium metabolic imaging." NMR Biomed 33.3(2020): e4235. 5. Feng Du, Shengping Liu,et al. "Numerical Simulation and Evaluation of a Four-Channel- by-Four-Channel Double-Nuclear RF Coil for 1HMRI and 31P MRSI at 7 T" IEEE Trans. Magn., vol. 54, no. 11, Nov. 2018, Art. No. 5101105Figures
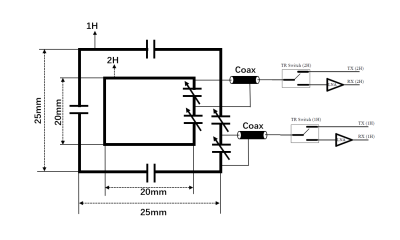
Fig. 1. Circuit diagram of the constructed
double- nuclear transceiver system
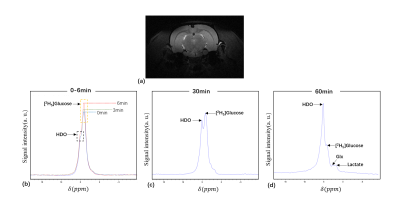
Fig. 2. (a) T2WI of a healthy rat brain; (b-d) 2H spectra
acquired from the healthy rat are shown at different
time points. The time points are labeled with different colors in (b).
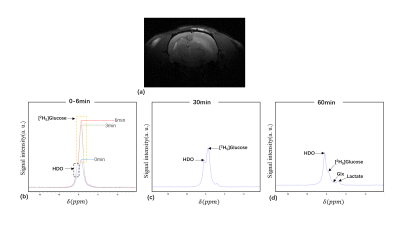
Fig. 3. (a) T2WI of a rat with glioma; (b-d) 2H spectra
acquired from one rat with glioma at different time points. The time points are
labeled with different colors in (b).

The
measured s-parameter the double- nuclear transceiver.

The
intensity of the natural abundance of 2H in six rat brains
DOI: https://doi.org/10.58530/2022/3243