3240
MR microscopy of human embryo at high isotropic spatial resolution1Graduate School of Science and Technology, University of Tsukuba, Tsukuba, Japan, 2Congenital Anomaly Research Center, Kyoto University Graduate School of Medicine, Kyoto, Japan, 3MRI simulations Inc., Minato-ku, Japan
Synopsis
High-resolution MR microscopy is advantageous for the human embryo study, but had not been realized because of many challenges. In this study, we demonstrated high-resolution MR microscopy of a human embryo specimen with 18 µm isotropic resolution. To achieve the higher spatial resolution than the previous system, we improved the hardware and optimized the pulse sequence. The MR images of the embryo showed detailed anatomical structures and were similar in appearance to the stained images obtained from an embryo specimen at the same developmental stage.
INTRODUCTION
High-resolution MR microscopy is advantageous for the human embryo study, but had not been realized because of many challenges. In this study, we demonstrated high-resolution MR microscopy of a human embryo specimen with 18 µm isotropic resolution. To achieve the higher spatial resolution than the previous system, we improved the hardware and optimized the pulse sequence. The MR images of the embryo showed detailed anatomical structures and were similar in appearance to the stained images obtained from an embryo specimen at the same developmental stage.METHOD
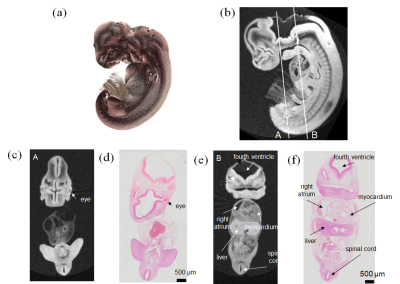
Embryo specimenWe measured a chemically-fixed human embryo specimen at Carnegie stage of 16 (crown-rump length = 8 mm) in an NMR sample tube (inner/outer diameter =8.8/10 mm) fixed with a 1 % agarose gel (Fig. 1(a)).
MR microscopy system
We utilized an MRI system consisting of a 9.4 T vertical wide bore (89 mm diameter) superconducting magnet (Oxford Instruments) (Fig. 1(b)), cylindrical gradients with a maximum gradient strength of 160 G/cm (Fig. 1(c)), and digital MRI console (DTRX6, MRTechnology, Japan). We constructed a solenoid RF coil with an inner radius of 10.4 mm and a length of 9 mm to closely fit a specimen (Fig. 1(d, e)). The shield was made of copper foil with a thickness of 0.1 mm, a height of 80 mm, and an outer diameter of 22 mm.
Imaging sequence
Flip angle dependence
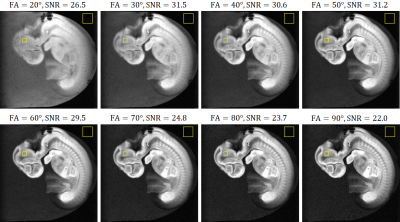
A steady-state free precession (SSFP) sequence (TR/TE = 100 ms/4 ms; FA = 20, 30, 40, 50, 60, 70, 80, and 90 degrees) was used with the matrix size of 256×256×16 and the voxel size of 36×36×576 µm3. The total image acquisition time was about 7 minutes.
High-resolution imaging
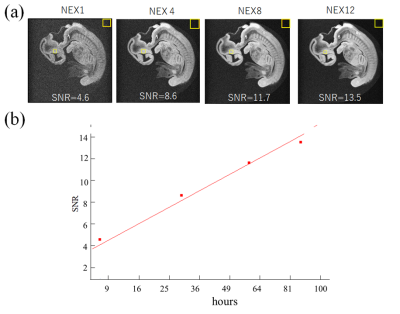
The same SSFP sequence was used for high-resolution imaging, except that the matrix size = (512) 3 and the voxel size = (18 µm)3. The number of excitations (NEXs) were 1, 4, 8, and 12, corresponding to the acquisition time of 7.3, 29,58, and 87 hours. We compared the high-resolution images with those obtained with the previous system [2] using a 3D gradient echo sequence with the following parameters: TR/TE = 100ms/5ms; FA= 90 degree, matrix size = 256×256×512, the voxel size = (40 µm)3, NEX = 12, and the acquisition time = 22 hours. The MRI system used in the previous study consisted of a 9.4 T vertical narrow bore (54 mm diameter) superconducting magnet (Oxford Instruments), a saddle RF coil with inner diameter of 8 mm, and cylindrical gradients with a maximum gradient strength of 48 G/cm. The embryo specimen was different but at the same developmental stage.
RESULTS
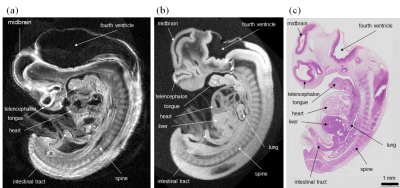
Figure 2 shows the sagittal images obtained with different FAs. The SNR was maximum when FA = 50°, and we chose this value for high resolution imaging. Figure 3 compares the high-resolution MR images obtained with the new and previous systems. The new system provided the clear image that was similar to stained images, whereas the image acquired with the old system showed blurring, distortion and other artifacts that obscured the distinction between different organs and tissues. Figure 4 shows the coronal images acquired with the new system, indicating the detailed structural information in the brain, liver, ventricle, spinal cord, and the other organs. Figure 5 shows the SNR values for the different NEXs. The SNR increased in proportion to the square root of NEX, almost following the theoretical equation.DISCUSSION
We have made the several updates to the previous system to allow for high resolution imaging; We constructed to the small solenoid to fit the specimen instead of using the birdcage coil. The MRI console was digitized [3] and the gradient system was upgraded to produce the higher gradient strength. There were many artifacts observed in the images obtained with the previous system, which would be caused by the hardware imperfection of the previous gradient system. These artifacts were not seen in the new system, possibly because of the hardware improvement. The pulse sequence was also optimized to obtain the high SNR. All these improvements enabled the high-resolution images of the human embryo at 18 µm isotropic resolution, which could visualize many organs and tissues with the high image quality.To further increase the spatial resolution, the SNR must be kept high enough within a realistic imaging time. In this study, the SNR for NEX=1 (7.3 hours of imaging time) was 4.6, which meets the noise criteria on the detectable resolution in MRI [4]. Given that SNR was proportional to the square root of NEX, when we double the spatial resolution (9 µm), the same SNR would be obtained with 117 hours of imaging. This indicates the possibility of higher-resolution imaging below 10 um.
Acknowledgements
No acknowledgement found.References
[1] Y. Matsuda, S. Ono, Y. Otake, S. Handa, K. Kose, T. Haishi, S. Yamada, C. Uwabe, Imaging of a Large Collection of Human Embryo Using a super-Parallel MR Microscope. Magn Reson Med Sci 6, 139-146 (2007).
[2] Y. Otake, S. Handa, K. Kose, K. Shiota, S. Yamada, C. Uwabe. Magnetic Resonance Microscopy of Chemically Fixed Human Embryos at High Spatial Resolution. Magn Reson Med Sci 14(2), 153-158 (2014).
[3] Seitaro Hashimoto, Katsumi Kose, Tomoyuki Haishi.Comparison of Analog and Digital Transceiver Systems for MR Imaging.Magn Reson Med Sci 13(4), 285-291 (2014).
[4] Rose A. Vision: human and electronic. (Optical physics and engineering.) New York: Plenum; 1973
Figures