3232
An 8-element torso RF coil array for the Australian inline MRI-Linac: Initial imaging results1School of Information Technology and Electrical Engineering, The University of Queensland, Brisbane, Australia, 2ACRF Image X Institute, The University of Sydney, Sydney, Australia, 3Ingham Institute, Sydney, Australia
Synopsis
The hybrid MRI-Linac system demands dedicated radiolucent imaging coils to visualise and track tumours without interfering the Linac beams. A radiolucent whole-body transceive RF coil for the Australian inline MRI-Linac system has been previously developed and produced images with good quality. Recently, an 8-element torso RF coil array was manufactured to provide better SNR with fast imaging capability, which will facilitate real-time tumour tracking during radiotherapy. Initial imaging results acquired from the 1.0 T Australian MRI-Linac system on a phantom and a volunteer demonstrate the potential benefits of such a torso coil array for MRI-guided radiotherapies.
Introduction
The MRI-Linac is a cutting-edge system that uses MRI for precise guidance of radiotherapy. This integrated system demands dedicated radiolucent RF coils for MR imaging without interfering the Linac beams. A radiolucent whole-body transceive RF coil for the Australian inline MRI-Linac system has been developed and produced images with good quality 1. However, an array of simultaneous receive coils can provide better SNR with fast imaging capability 2,3, to enable real-time tumour tracking during radiotherapy. We have previously proposed such an array consisting of eight surface receive coils 4. Recently, we manufactured this coil array and acquired images on the 1.0 T Australian MRI-Linac system located at the Liverpool Hospital (Sydney, Australia). Initial imaging results on a phantom and a volunteer are shown in this abstract to demonstrate the benefits of such a torso coil array for MRI-guided radiotherapies.Methods
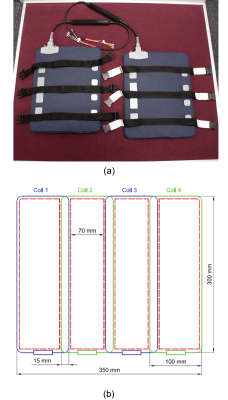
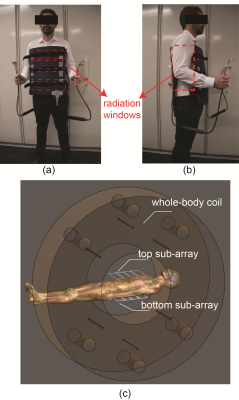
As shown in the Figure 1 (a), the 8-element coil array consists of two 4-element sub-arrays, which can be attached to the patient with an integrated adjustable strap-system. The coil-patient setup and radiation windows are shown in Figure 2 (a) and (b) and it is compatible to the developed rotating couch5 for the MRI-Linac system. The coil-patient setup within the Australian MRI-Linac is demonstrated in Figure 2 (c) with the simulated MRI-Linac whole body coil and the Duke model. The internal topology of each sub-array is elaborated in Figure 1 (b). The length and width of the individual coil element is 300 mm and 100 mm, respectively. The width of overlapped decoupling area is 15 mm, extra decoupling networks were used to decouple the next-neighbouring coils, such as coil 1 and coil 3 in Figure 1(b). The adjacent (e.g. S12) and next neighbouring (e.g. S13) bench-measured decoupling was better than -18 dB and -24dB, respectively. The S11 of individual channels was better than -32 dB.The loop coil was made of thin flexible transmission line (Φ: 1.87mm), which can help to maximise the size of the anterior/posterior radiation windows and allowed for optimal fitting to the patient for different imaging setups. As shown in the Figure 1 (b) and Figure 2(a), the 70 mm wide anterior/posterior radiation windows were created between the conductors of the coils and the bilateral radiation windows were created between two sub-arrays (Figure 2(b)). The numerical simulation of the coil array and a water phantom was also used to produce simulated images to be compared against experiment images. The coil array was simulated with the Finite-difference time-domain (FDTD)6 based electromagnetic simulation software Sim4Life (ZMT, Zurich, Switzerland) with the Duke human model from the Virtual Family 7. The relative permittivity and conductivity of the water phantom was set to 78 and 0.5 S/m in the simulation. A 20 L phantom was formulated with 1.24 g NiSO4 × 6 H2O and 2.62 NaCl per 1000 mL distilled water. All data were acquired on the 1.0 T Australian MRI-Linac system located at the Liverpool Hospital (Sydney, Australia) with the Siemens MRI console (Siemens Healthcare, Erlangen, Germany). For SNR analysis, the noise images were produced by setting the excitation voltage of the GRE sequence to 0 volt.
Results
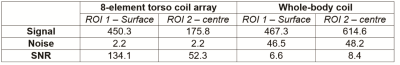
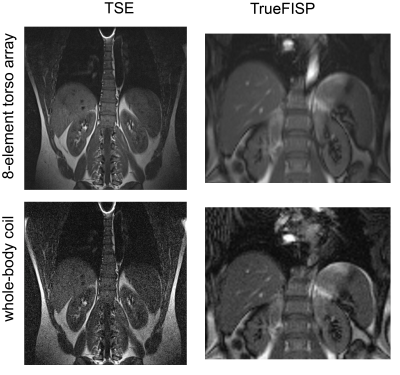
As shown in Figure 3 (a), the sensitivity weighting of the simulated image is comparable to the phantom image acquired in the experiment (Figure 3(b)). Compared to the image acquired with the whole-body coil (Figure 3(c)), the image acquired with the torso coil array has much lower visual noise and higher image quality. The detailed comparison among signal, noise and SNR are listed in Table I. The noise was evaluated from the acquired 0-volt noise image and Figure 3 (b) and (c) were produced with the same excitation voltage (320V). Results show when using the 8-element surface coil array, the SNR at the surface (ROI 1) and centre (ROI 2) has 20 times and 5 times increase compared with the whole-body coil. The in vivo kidney images acquired with TSE and TrueFISP sequences are shown in Figure 4. The 8-element torso coil array can produce images with lower noise and higher SNR that will be beneficial for tumour segmentation and tracking in radiotherapy 8. The improved SNR can be also used as a leverage to achieve higher scan-time reduction, while performing parallel imaging and compressed sensing.Conclusion
In this work, we have shown initial imaging results on a phantom and a volunteer and characterised a custom built 8-element torso coil array for the Australian 1.0T inline MRI-Linac system. The torso coil array demonstrated its superiority over the whole-body coil with 5~20 times higher SNR and 20 times lower noise. High quality images acquired with this torso coil array will facilitate MR-guided radiotherapy with improved accuracy for tumour segmentation and real-time tumour tracking.Acknowledgements
This work is supported by the National Health and Medical Research Council of Australia for the project "The Australian MRI-Linac Program: Transforming the Science and Clinical Practice of Cancer Radiotherapy." The authors wish to thank ZMT Zurich MedTech for providing the simulation software package Sim4Life.References
1. Liney GP, Dong B, Weber E, et al. Imaging performance of a dedicated radiation transparent RF coil on a 1.0 Tesla inline MRI-linac. Phys Med Biol. Jun 25 2018;63(13):135005. doi:10.1088/1361-6560/aac813
2. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magnetic resonance in medicine. Nov 1999;42(5):952-62.
3. Griswold MA, Jakob PM, Heidemann RM, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic resonance in medicine. Jun 2002;47(6):1202-10. doi:10.1002/mrm.10171
4. Mingyan Li EW, Aurelien Destruel , Feng Liu , and Stuart Crozier. Theoretical Design and Image Reconstruction of an 8-element Receive-only Surface Coil Array for the 1.0 T Australian MRI-Linac System. 2020:
5. Yee K. Numerical solution of initial boundary value problems involving maxwell's equations in isotropic media. Antennas and Propagation, IEEE Transactions on. 1966;14(3):302-307.
6. Gosselin M-C, Neufeld E, Moser H, et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: the Virtual Population 3.0. Physics in Medicine and Biology. 2014/08/21 2014;59(18):5287-5303. doi:10.1088/0031-9155/59/18/5287
7. Buckley JG, Dong B, Liney GP. Imaging performance of a high-field in-line magnetic resonance imaging linear accelerator with a patient rotation system for fixed-gantry radiotherapy. Phys Imaging Radiat Oncol. Oct 2020;16:130-133. doi:10.1016/j.phro.2020.11.001
8. Liu PZY, Dong B, Nguyen DT, et al. First experimental investigation of simultaneously tracking two independently moving targets on an MRI-linac using real-time MRI and MLC tracking. Medical Physics. 2020;47(12):6440-6449. doi:https://doi.org/10.1002/mp.14536
Figures