3209
Modular Graphene Quantum Dot Nano-transformers: An Efficient and Versatile Vehicle for Localized Tumor Imaging and Treatment1Key Laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences–Wuhan National Laboratory for Optoelectronics, Wuhan, China
Synopsis
A versatile nano-vehicle, quantum dots nano-transformers, smartly transforms its shape in acidic tumor microenvironment to prolong the tumor retention, and then disassembles and releases the encapsulated photosensitizers to “turn on” the imaging contrast. The total drug dose in repeated photodynamic therapy is reduced because one injection is enough for four times of light irradiation.
Introduction
Imaging guided photodynamic therapy (PDT) is considered as an emerging therapeutic modality against cancer with high spatiotemporal selectivity because it can combine the advantages of both diagnosis and treatment simultaneously.[1] To maximize the efficacy, the process is usually applied repetitively for ablating various tumors.[2] However, the total overdose of contrast agents (CAs) and photosensitizers (PSs) from repeated administrations causes severe side effects.[3] Herein, we develop acidity-activated graphene quantum dots nano-transformers (GQD NT) as CAs and PSs vehicles for long-period tumor T1-MR/FL imaging and repeated PDT.Methods
GQD NT were easily formed by self-assembly of the targeting module, loading module and linking module at room temperature, and CAs/PSs were simultaneously encapsulated during the host-guest interaction process between β-cyclodextrin and adamantine. To evaluate the potential use of GQD NT for in vivo imaging and tumor therapy, we performed fluorescence/magnetic resonance imaging and PDT of A549 tumor-bearing mice intravenously injected with GQD NT-encapsulated TCPP and Mn(II)-TCPP.Results and Discussion
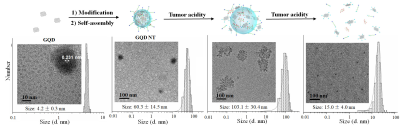
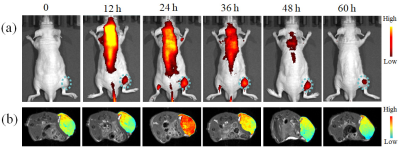
Through the host-guest interaction between the target module, loading module and the linking module, the free GQD with an average diameter of 4 nm were self-assembled to GQD NT with a relatively uniform diameter of approximately 60 nm (Figure 1). The acidic tumor extra-cellular pH could trigger the transformation of tight GQD NT to a loosen state and increased the nanoparticle diameter to 103 nm, which was favorable for retaining in tumor areas. When time prolonged to 24 h, the GQD NT was dissociated, and the diameter decreased to 15 nm. Such a small size was beneficial to the efficient extravasation through tumor vascular fenestrations, deep penetration and easy incorporation of nanoparticles into cancer cells.Figure 2a illustrated the in vivo biodistribution profile and tumor accumulation of TCPP@GQD NT in A549 tumor-bearing mice. Fluorescent imaging showed that the PSs were maintained in the tumor for over 60 h. This is beneficial for subsequent PDT because the longer retention time provides more opportunities for laser irradiation. T1-weighted MRI revealed obviously increased tumor contrast 24 h after Mn-TCPP@GQD NT injection (Figure 2b), and the longitudinal relaxation time of the tumor decreased from 2.7 s to 1.9 s.
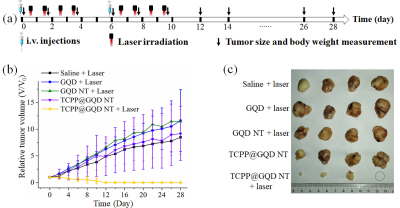
We further evaluated the in vivo PDT efficiency of GQD NT. For TCPP@GQD NT with laser irradiation group, the tumor area was irradiated with a 650 nm laser on four consecutive days 12 h after drug administration (Figure 3a). The treatment was repeated once. Two weeks after treatment, the mice treated with TCPP@GQD NT and laser irradiation showed delayed tumor growth (Figure 3b) and no tumor recurrence was observed in the following 2 weeks (Figure 3c).
Acknowledgements
This work is supported by National Key R&D Program of China (2018YFA0700400), National Natural Science Foundation of China (91859206, 81625011, 81871453, 21921004).References
[1] Li Z, Li S, Guo Y, et al. Metal-free nanoassemblies of water-soluble photosensitizer and adenosine triphosphate for efficient and precise photodynamic cancer therapy. ACS Nano 2021;15(3):4979‐4988.
[2] An J, Hu Y, Li C, et al. A pH/ultrasound dual-response biomimetic nanoplatform for nitric oxide gas-sonodynamic combined therapy and repeated ultrasound for relieving hypoxia. Biomaterials 2020;230:119636.
[3] Brown S B, Brown E A, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5(8):497‐508.
Figures