3181
Using the Artificial Intelligence Based Compressed SENSE Technology in Acute Cerebral Infarction1Department of Radiology, The first hospital of jilin university, Changchun,Jilin, China, 2Philips Healthcare, Beijing, China
Synopsis
MRI has advantages in detecting acute ischemia and describing the core volume of the infarct without radiation, but the long scanning time affects clinical use. Compressed sense combined with artificial intelligence (CS-AI) technology accelerates the acquisition of imaging. This study aims to use the CS-AI technique to accelerate common sequences of Acute cerebral infarction and investigate the effect of different acceleration factors on image quality and diagnosis. The results show that CS-AI reconstruction scans reduce scanning time while maintaining image quality compared to conventional SENSE.
Introduction
Acute ischemic stroke is the most common type of stroke, with a high prevalence, disability, and mortality rate. MRI plays a vital role in detecting acute ischemia and depicting core volumes of infarcts without radiation. 1 However, the long acquisition time of MRI limits its use in clinical settings. Philips’ commercial compressed-SENSE(CS) technology allows shortening of scan time and provides comparable overall image quality.2 In recent years, with the rise of artificial intelligence (AI) technology, integrating deep neural networks into MRI rebuilding and instead of traditional iterative reconstruction has become a reality and has already demonstrated excellent performance. Furthermore, the CS-AI technique has been expanded to multiple commonly scanning sequences and anatomical areas. The purpose of this study was to acquire highly accelerated acute cerebral infarction imaging in different sequences using the CS-AI reconstruction and compare the image quality with the conventional SENSE, CS and CS-AI with different acceleration factors (AF).Materials and Methods
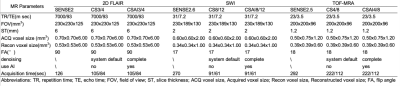
In a prospective study, a total of 6 patients with acute cerebral infarction (mean age 59.5 years, age range 49-73 years) was enrolled. After obtaining informed consent, three sequences included 2D FLAIR, SWI and TOF-MRA were acquired on a 3.0T Philips Elition scanner. The sequence scan parameters are shown in Table 1. After pre-experimented on the water model, different acceleration factors were designed for each sequence, with AF 3 and 4 for 2D FLAIR, AF 8 and 12 for SWI, and AF 4 and 8 for TOF-MRA. Each sequence was reconstructed by CS and CS-AI. Moreover, we also scanned the conventional parallel imaging for 2D FLAIR, SWI, and TOF-MRA (SENSE, AF = 2, 2.5 and 2.6), which are the parameters recommended by the MRI vendor. The images were transferred to the Philips IntelliSpace Portal. Signal to noise ratio (SNR) and contrast to noise ratio (CNR) were calculated by setting the region of interest(ROI) at lesion(ROI1) and adjacent white matter(ROI2) (or in the absence of the lesion, on areas of normal parietal lobe), M1 segment of the middle cerebral artery on the healthy side(ROI1) and temporalis muscle(ROI2). According to Equation 1 and Equation 2 to calculate SNR and CNR. Two radiologists with 3 and 5 years’ experience respectively evaluate the quality of the images on a 3-point scale, including deep nuclei, grey-white matter demarcation, and vascularity (rules: 1-indistinguishable; 2-blurring; 3-clear). The agreement between the two reviewers on the evaluation of qualitative information was tested by the Kappa agreement test, with a kappa value >0.6 being considered a good agreement. The quantitative image quality results were analyzed using the Friedman test and post-hot analysis, and differences were considered statistically significant at P<0.05.Equation 1: $$SNR_{tissue1}=\frac{SI_{ROI1}}{SD_{ROI1}}$$
Equation 2: $$CNR_{tissue1-tissue2}=\frac{|SI_{ROI1}-SI_{ROI2}|}{\sqrt{SD_{ROI1}^{2}+SD_{ROI2}^{2}}}$$
Results
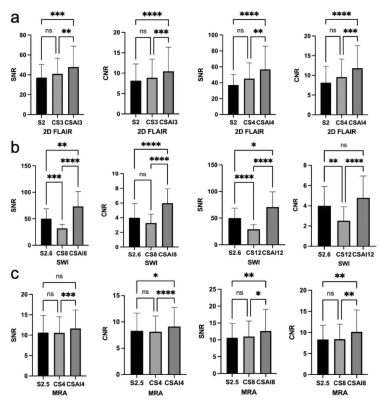
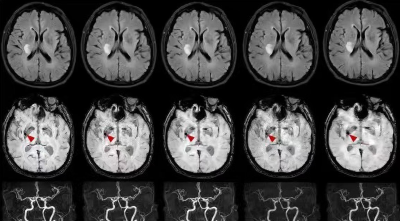
The subjective image quality assessment of the two separate radiologists was highly consistent, with K=0.708, K=0.897 and K=0.822 for the three sequences 2D FLAIR, SWI and TOF-MRA respectively. Figure2(a-c) shows a schematic representation of some typical results. For 2D FLAIR, the SNR and the CNR of CSAI3 were significantly higher than the reference (P=0.0005, P<0.0001), as well as between CSAI4 and the reference (both P<0.0001). For SWI, a significant difference was found between the SNR and CNR of CSAI8 and the reference (P=0.0065, P<0.0001), the SNR of CSAI12 had a significant difference than the reference(P=0.04), while there was no significant difference in CNR between the CSAI12 and the reference (P=0.178). For TOF-MRA, SNR was comparable with CSAI4 and the reference(P=0.178), but the CNR of CSAI4 shows a significant difference from the reference(P=0.04). The SNR of CSAI8 was statistically higher than the reference scan(P=0.0096), as was the CNR(P=0.0044). Figure 3 shows the comparison of 2D FLAIR, SWI and TOF-MRA images with different acceleration methods and acceleration factors.Discussion
Three sequences of 2D FLAIR, SWI and TOF-MRA were used in this study. 2D FLAIR was used to detect the extent of the lesion and is known to have a higher contrast of the lesion than T2WI. SWI is widely used in the detection of hemorrhage due to its sensitivity to blood breakdown products.3 MRA detects the presence and location of vascular occlusions without the use of a contrast medium. This paper is the first to apply CS-AI to the diagnosis of cerebral infarction on magnetic resonance imaging. The results of this paper show that compared to SENSE and CS, CS-AI significantly reduces the noise. However, for SWI, as the acceleration factor increases to 12, SNR and CNR decrease. It may be due to the fact that when accelerated to a certain point, too few data points were collected during k-space undersampling. One of the patients included in this study had a microbleed detected on SWI, even at an acceleration factor of 12, which did not affect the detection of this lesion. In addition, the effect of the CS-AI application on the perfusion sequence could not be evaluated for hardware reasons and will continue to be assessed in the next step. At the same time, for the selection of acceleration factors, the view of this paper is consistent with Molnar et al, the appropriate acceleration factor is chosen for each sequence.4Acknowledgements
No acknowledgement found.References
1. Nael, K. et al. Six-Minute Magnetic Resonance Imaging Protocol for Evaluation of Acute Ischemic Stroke: Pushing the Boundaries. Stroke 45, 1985–1991 (2014).
2. Vranic, J. E. et al. Compressed Sensing–Sensitivity Encoding (CS-SENSE) Accelerated Brain Imaging: Reduced Scan Time without Reduced Image Quality. AJNR Am J Neuroradiol 40, 92–98 (2019).
3. Li, L. et al. Susceptibility-weighted Imaging in Thrombolytic Therapy of Acute Ischemic Stroke. Chin Med J (Engl) 130, 2489–2497 (2017).
4. Molnar, U. et al. Diagnostic quality assessment of compressed SENSE accelerated magnetic resonance images in standard neuroimaging protocol: Choosing the right acceleration. Phys Med 88, 158–166 (2021).
Figures