3178
Prediction of Drug Treatment Outcome among Epilepsy Children with Tuberous Sclerosis Complex based on Deep Neural Network and Multi-contrast MRI1Research Centre for Medical AI, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2University of Chinese Academy of Sciences, Beijing, China, 3Department of Neurology, Shenzhen Children’s Hospital, Shenzhen, China, 4Department of Radiology, Shenzhen Children’s Hospital, Shenzhen, China, 5Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
Synopsis
Distinguishing epilepsy drug treatment outcomes is crucial for treating children with tuberous sclerosis complex (TSC). Here, a deep-learning framework named AE-net was proposed to analyze epilepsy drug treatment outcomes using multi-contrast MRI data. Firstly, multi-contrast image-based models were respectively generated using the EfficientNet3D-B0 networks. Then, an averaging ensemble network was created as the final model. The proposed AE-net achieved the best AUC performance of 0.800 and sub-optimal AUC performance of 0.763 in the testing cohort, better than others. And the proposed method can predict epilepsy drug treatment outcomes to help clinical radiologists formulate more targeted treatments in the future.
Introduction
Epilepsy is the most challenging manifestation of tuberous sclerosis complex (TSC), affecting approximately 85% of patients1. The classic treatment for epilepsy is anti-epileptic drugs (AEDs)2, but over 50% of patients with TSC develop drug resistance3. Therefore, distinguishing refractory patients from seizure-controlled patients is an urgent need in the clinic.Since now, multi-contrast MRI data are mainly used to predict epilepsy drug treatment outcomes4. However, this diagnosis was still manual. Otherwise, the deep neural network is an advanced technology, which can extract features automatically from data and perform characterization learning based on data. Previous studies have shown the ability of convolutional neural network (CNN) to detect cortical tubers in TSC5 and classify lung cancer and bone lesions on MRI with high accuracy 6,7.
In this study, we proposed a deep-learning method to differentiate epilepsy drug treatment outcomes in children with TSC. The experiments had shown that the proposed method could successfully differentiate epilepsy drug treatment outcomes in children with TSC.
Methods
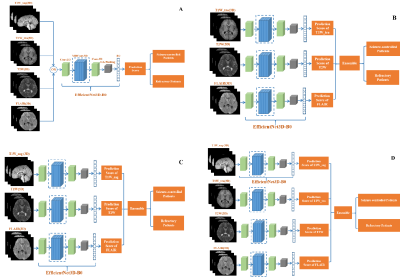
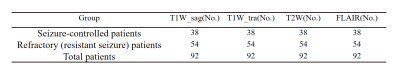
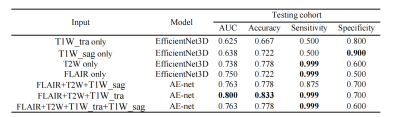
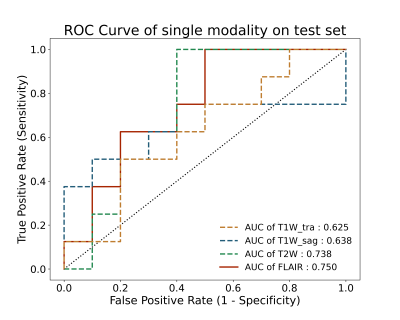
The proposed method was based on the EfficientNet architecture8, which was a state-of-the-art classification network. The proposed model is an improvement upon previously developed EfficientNet designs. The MRI image-based models were the 3D version of Network modified on EfficientNet-B08. The image-based models were combined using an averaging ensemble approach named AE-net, which received prediction scores from the image-based models as inputs and created outputs based upon a mean of the prediction scores.For the drug treatment outcome classification tasks with one single input modality, the EfficientNet3D-B0 architecture was applied (Figure 1A). When AE-net was used, three options were experimented with for information fusion. First, three transverse modality data of transverse T1W (T1W_tra), T2W, and FLAIR were used as inputs (Figure 1B). Second, we replaced T1W_tra with sagittal T1W (T1W_sag) (Figure 1C). Finally, we use all four sequences as network inputs (Figure 1D).
The IRB-approved data were obtained from 92 TSC-related epilepsy patients who had anti-epileptic drug (AED) treatments for at least one year and consisted of T1W_tra, T1W_sag, T2W, and FLAIR before AED treatment (Table 1). Data were randomly split into a training dataset (n = 74) and an independent test dataset (n = 18). Drug treatment outcomes were defined according to the 1981 ILAE classification9, which were recorded as a seizure-controlled or refractory (resistant seizure) group. Patients were considered as the controlled if they did not have clinical seizures for at least 1 year. Refractory patients had at least one seizure in the past year or death.
In neuroimaging studies, most of the lesions are located in the brain tissue. Therefore, we first removed the unrelated non-brain tissues in MRI using a deep-learning tool HD-bet10. The size of all 3D MRI images was resized to (128,128,128), and the image intensity was then normalized to the range between 0 and 1.
Models were trained with a learning rate of 0.001, batch size of 4 for 50 epochs, Adam optimization, and the loss function of binary cross-entropy. Five-fold cross-validation was used to evaluate the models. The area under the receiver operating curve (ROC) (AUC) of cross-validation as the metric for model evaluation during training, while preserving the model achieved the best level of AUC during training. Here, AUC, accuracy, sensitivity, and specificity were calculated to assess the classification performance for each model in each cohort. Models’ train, validation, and test were implemented with python (version 3.8.10) and PyTorch (version 1.9.0) environment.
Results
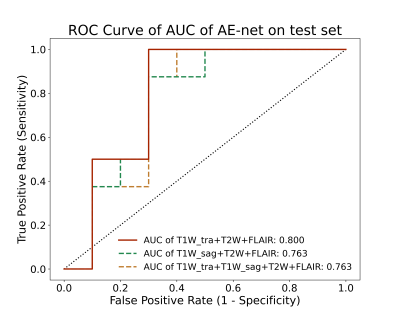
Performance of the single input modality of EfficientNet3D-B0 and proposed AE-net models on the dependent test set described in Table 1. The best classification results were obtained for the proposed AE-net model (FLAIR+T2W+T1W_tra) with an AUC of 0.800 in the testing cohort. In our proposed AE-net, replacing T1W_tra with T1_sag and using all four sequences also achieved a sub-optimal AUC performance of 0.763. Figure 2 shows the ROC curve for the single input modality of EfficientNet3D-B0 models’ performance on the test set. Figure 3 shows the ROC curve for our proposed AE-net models’ performance on the test set.Discussion and Conclusion
In this study, the proposed AE-net with three transverse modality data achieved the best AUC performance of 0.800, which indicates that integrating multiple-contrast data from multiple sequences on MRI scanners is better than a single MRI sequence, and the same data collection direction allowed the most efficient grasp of the data distribution and eventually yielded the best classification performances. The current test results have suggested that the proposed method could be a non-invasive, efficient, and reliable way to predict drug treatment outcomes in TSC patients. In the future, more studies should include larger sample size, preferably from multiple centers and a weighted ensemble can be used for sequence integration.Acknowledgements
Some of the work was partially supported by the Sanming Project of Medicine in Shenzhen (SZSM201812005), the Pearl River Talent Recruitment Program of Guangdong Province (2019QN01Y986), Shenzhen Science and Technology Program (JCYJ20210324115810030) and the National Natural Science Foundation of China (61871373, 81729003, and 81901736).References
1.P. Curatolo et al., "Management of epilepsy associated with tuberous sclerosis complex: Updated clinical recommendations," Eur J Paediatr Neurol, vol. 22, no. 5, pp. 738-748, Sep 2018, doi: 10.1016/j.ejpn.2018.05.006.
2.E. van der Poest Clement et al., "Update on Drug Management of Refractory Epilepsy in Tuberous Sclerosis Complex," Paediatr Drugs, vol. 22, no. 1, pp. 73-84, Feb 2020, doi: 10.1007/s40272-019-00376-0.
3.S. Jesmanas et al., "Different MRI-defined tuber types in tuberous sclerosis complex: Quantitative evaluation and association with disease manifestations," Brain Dev, vol. 40, no. 3, pp. 196-204, Mar 2018, doi: 10.1016/j.braindev.2017.11.010.
4.J. Yang et al., "Machine Learning in Epilepsy Drug Treatment Outcome Prediction Using Multi-modality Data in Children with Tuberous Sclerosis Complex," presented at the 2020 6th International Conference on Big Data and Information Analytics (BigDIA), 2020.
5.I. Sanchez Fernandez et al., "Deep learning in rare disease. Detection of tubers in tuberous sclerosis complex," PLoS One, vol. 15, no. 4, p. e0232376, 2020, doi: 10.1371/journal.pone.0232376.
6.F. R. Eweje et al., "Deep Learning for Classification of Bone Lesions on Routine MRI," EBioMedicine, vol. 68, p. 103402, Jun 2021, doi: 10.1016/j.ebiom.2021.103402.
7.R. Grossman et al., "Differentiating Small-Cell Lung Cancer From Non-Small-Cell Lung Cancer Brain Metastases Based on MRI Using Efficientnet and Transfer Learning Approach," Technol Cancer Res Treat, vol. 20, p. 15330338211004919, Jan-Dec 2021, doi: 10.1177/15330338211004919.
8. M. Tan and Q. Le, "Efficientnet: Rethinking model scaling for convolutional neural networks," in International Conference on Machine Learning, 2019: PMLR, pp. 6105-6114.
9.S. Shinnar, "The new ILAE classification," (in English), Epilepsia, vol. 51, no. 4, pp. 715-717, Apr 2010, doi: DOI 10.1111/j.1528-1167.2010.02542.x.
10.F. Isensee et al., "Automated brain extraction of multisequence MRI using artificial neural networks," (in English), Hum Brain Mapp, vol. 40, no. 17, pp. 4952-4964, Dec 1 2019, doi: 10.1002/hbm.24750.
Figures