3167
Tracking forearm muscle fibers from Diffusion MRI during dynamic contractions1Shanghai Jiao Tong University, Shanghai, China, 2MR Collaborations, Siemens Healthineers Ltd., Shanghai, China, 3Siemens Digital Medical Technology (Shanghai) Co., Ltd., Shanghai, China, 4Huashan Hospital affiliated to Fudan University, Shanghai, China
Synopsis
To reconstruct the trajectories of forearm muscle fibers during dynamic contractions, we designed a customized MRI-compatible rig to help acquire MRI data of the forearm muscles under three wrist postures. A piecewise registration method was proposed to correct the misregistration between DTI and anatomical images. The reconstructed supinator was highly consistent with the cadaveric dissection reference. Diffusion and architectural parameters were calculated to characterize voluntary contractions. During the wrist flexion and extension, the fascicle length of the supinator was inversely related to the pennation angle. The diffusion parameters increased slightly from neutral position to the other two postures.
Introduction
DTI has been applied to track white matter bundles1 and assess muscle fiber directions2. The architecture of human muscles could be measured and visualized in vivo using DT-MRI, which enables researches on force generation and accurate physiological models3, 4. Previous studies on investigating muscle architectures using DTI mainly focused on large muscles in lower limbs5. A few studies turned to forearm muscles and designed MRI protocols to reveal these muscles’ complex arrangements6. In these studies, fiber tracking was performed without voluntary contractions due to the long scan time and the lack of MRI-compatible devices to immobilize the forearm7. Therefore, forearm muscle properties during voluntary isometric contractions haven’t been reported. Moreover, image post-processing is lacked for dynamic contractions and requires to be further elaborated. We acquired T2w images and DTI data of the forearm with a customized rig under three wrist postures. Simultaneous multi-slices (SMS) technique was used to acquire high-resolution MRI in reduced scan time. An optimized piecewise registration method was introduced into the post-processing to register the DTI data to the T2w images.Methods
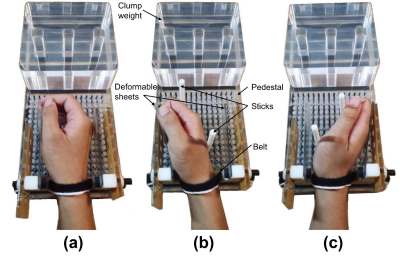
MRI data were collected from the right forearms of 4 healthy subjects (age: 25.7±1.5 years; 50% m) with a 3T scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using an 18-channel flex body coil. An MRI-compatible rig was designed to support the hand for wrist at neutral position, flexion, and extension during image acquisition (Figure 1). Deformable sheets were used to measure joint angle and to estimate the force applied by the subjects (3.3±0.8N, 18.3±4.6deg). T2w images were acquired using SMS technique (TSE sequence, TR/TE 3500/39 ms, acquisition matrix 448x448, transverse direction, voxel size 0.27x0.27x3mm3, SMS factor 2, scan time 216s). DTI images were under (EPI sequence, TR/TE 5500/69 ms, acquisition matrix 80x160, sagittal direction, voxel size 1.6x1.6x3 mm3, b value = 400s/mm2, diffusion directions 12, scan time 292s). Histogram-based N4 was applied for both T2w and DTI data to correct the bias field. Rician noise was removed from the DTI data through the LPCA filter8. Eddy current distortions were corrected using FSL 6.0. We proposed a piecewise registration method to register the DTI data to the T2w images. The two sequences were aligned along axial direction based on the bone markers around the wrist, and were then divided into five sections. The DTI data were registered with the anatomical images separately in each section using an affine transformation. The slices between adjacent sections were manually adjusted for consistency. Diffusion tensors were calculated using DSI Studio9. A range of 3-14 ROIs of the 13 muscles in the forearm were delineated in T2w images. Seed points were generated by using the farthest point sampling (FPS)10 with a fiber density of 350/mm2.Data-analysis
Two muscle architectural parameters were obtained from the results of fiber trajectories, including fascicle length (FL) and pennation angle (PA)11. Four diffusion parameters were calculated (axial diffusivity (AD = first eigenvalue), radial diffusivity (RD), mean diffusivity (MD) and anisotropy fraction (FA)).Results
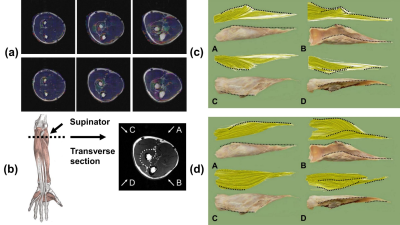
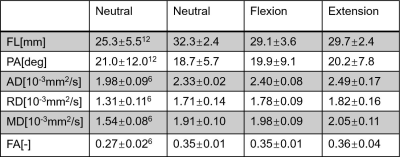
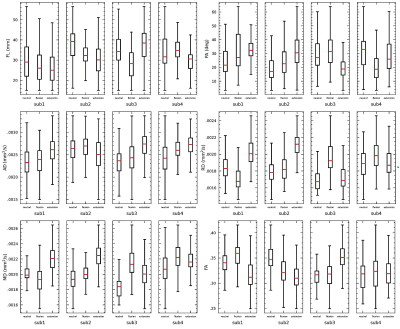
The registration results between the principal eigenvector maps and the anatomical images are displayed in Figure 2(a). The severe misregistration by transforming the whole series was corrected by using the proposed piecewise registration. Fiber trajectories of the supinator of a representative subject were in good correspondence with the cadaveric specimens (Figure 2(d)). The diffusion and architectural parameters of the supinator are compared with the parameters derived in previous studies6,12, which showed a similar distribution (Table 1). The variance in the parameters of individual subjects is displayed in Figure 3.Discussion
This study reconstructed the fiber trajectories in the forearm during dynamic contractions by improving data collection and post-processing. An MRI-compatible rig was designed to help subjects keep their voluntary contractions with least involuntary movements, as well as to measure isometric forces applied by the subjects. SMS technique also contributed to the data collection by providing high resolution images with reduced acquisition time. Piecewise registration was introduced to correct the misregistration from DTI to T2w due to the anisotropy of forearm anatomies. The regions indicated by the principal eigenvector maps after registration were highly consistent with the ROIs. The fiber trajectories of the supinator using the proposed method showed high consistency with the cadaveric specimens.The mean values of the diffusion and architectural parameters of the supinator in neutral position were slightly different from previous studies while the distribution was similar. The parameters of supinator did not variate much among the three postures, probably due to that supinator is not the dominant muscle to flex or extend the wrist. The decrease in FL and increase in PA13 of subject 1 and 2 in wrist flexion and extension may result from the great contraction force (4.5N, 3.3N). The diffusion parameters under wrist contractions were higher than in neutral position, which is consistent with the increasing diffusion parameters after exercise14. This phenomenon indicates that diffusion parameters could serve as an alternative of architectural parameters to reflect the micro changes during muscle contractions.
Conclusion
The reconstructed trajectories of the forearm muscle fibers are highly correlated with real anatomical structures and muscle contraction characteristics, indicating that the customized MRI-compatible rig and the piecewise registration method would help the study of dynamic muscle contractions.Acknowledgements
This work was supported in part by the China National Key R&D Program (Grant No. 2018YFB1307202), the National Natural Science Foundation of China (Grant No. 91948302, No. 51905339).
References
1. Lazar M, Weinstein DM, Tsuruda JS, Hasan KM, Arfanakis K, Meyerand ME, et al. White matter tractography using diffusion tensor deflection. Human Brain Mapping. 2003;18(4):306-21.
2. Heemskerk AM, Strijkers GJ, Vilanova A, Drost MR, Nicolay K. Determination of mouse skeletal muscle architecture using three-dimensional diffusion tensor imaging. Magnetic Resonance in Medicine. 2005;53(6):1333-40.
3. Ramasamy E, Avci O, Dorow B, Chong S-Y, Gizzi L, Steidle G, et al. An Efficient Modelling-Simulation-Analysis Workflow to Investigate Stump-Socket Interaction Using Patient-Specific, Three-Dimensional, Continuum-Mechanical, Finite Element Residual Limb Models. Frontiers in Bioengineering and Biotechnology. 2018;6.
4. Botelho DP, Curran K, Lowery MM. Anatomically accurate model of EMG during index finger flexion and abduction derived from diffusion tensor imaging. Plos Computational Biology. 2019;15(8).
5. Bolsterlee B, Finni T, D'Souza A, Eguchi J, Clarke EC, Herbert RD. Three-dimensional architecture of the whole human soleus muscle in vivo. Peerj. 2018;6.
6. Froeling M, Nederveen AJ, Heijtel DFR, Lataster A, Bos C, Nicolay K, et al. Diffusion-tensor MRI reveals the complex muscle architecture of the human forearm. Journal of Magnetic Resonance Imaging. 2012;36(1):237-48.
7. Levin DIW, Gilles B, Maedler B, Pai DK. Extracting skeletal muscle fiber fields from noisy diffusion tensor data. Medical Image Analysis. 2011;15(3):340-53.
8. Manjon JV, Coupe P, Concha L, Buades A, Collins DL, Robles M. Diffusion Weighted Image Denoising Using Overcomplete Local PCA. Plos One. 2013;8(9).
9. Yeh F-C, Verstynen TD, Wang Y, Fernandez-Miranda JC, Tseng W-YI. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. Plos One. 2013;8(11).
10. Konstantin A, Yu T, Le Carpentier E, Aoustin Y, Farina D. Simulation of Motor Unit Action Potential Recordings From Intramuscular Multichannel Scanning Electrodes. Ieee Transactions on Biomedical Engineering. 2020;67(7):2005-14.
11. Lee D, Li Z, Sohail QZ, Jackson K, Fiume E, Agur A. A three-dimensional approach to pennation angle estimation for human skeletal muscle. Computer Methods in Biomechanics and Biomedical Engineering. 2015;18(13):1474-84.
12. Li Z, Mogk JPM, Lee D, Bibliowicz J, Agur AM. Development of an architecturally comprehensive database of forearm flexors and extensors from a single cadaveric specimen. Computer Methods in Biomechanics and Biomedical Engineering-Imaging and Visualization. 2015;3(1):3-12.
13. Bolsterlee B, D'Souza A, Gandevia SC, Herbert RD. How does passive lengthening change the architecture of the human medial gastrocnemius muscle? Journal of Applied Physiology. 2017;122(4):727-38.
14. Froeling M, Oudeman J, Strijkers GJ, Maas M, Drost MR, Nicolay K, et al. Muscle Changes Detected with Diffusion-Tensor Imaging after Long-Distance Running. Radiology. 2015;274(2):548-62.
Figures