3162
CE-MRI Based Radiomics Model to identify Crohn’s disease and Ulcerative colitis1Magnetic Resonance, Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi’an, China
Synopsis
Diagnosis and differential diagnosis of Inflammatory Bowel Disease (IBD) remains challenge since the particularity of small bowel. The objective of this study was to use the contrast enhanced MRI (CE-MRI) radiomics to distinguish subtypes of IBD patients. A total of 216 patients confirmed by pathology underwent small bowel CE-MRI. After the pipeline of radiomics, it was found that wavelet transformed texture features can effectively identify ulcerative colitis (UC) and Crohn’s disease (CD) with high performance. This work provides more detailed and microscopic details for the differential diagnosis of IBD subtypes based on the preliminary results.
Introduction
Inflammatory bowel disease (IBD) is a group of non-specific chronic and recurrent inflammatory diseases of the bowel, characterized by abnormal intestinal edema and thickening caused by immune response1. The diagnosis and differential diagnosis of Crohn’s disease (CD) and ulcerative colitis (UC), is often challenging due to the limitations of small bowel visualization2. Traditional diagnostic methods of either CD or UC are established based on the clinical manifestation, laboratory tests, imaging techniques, endoscopy, and histopathology results3. The specific objective of this study was to explore the radiomics features based on MR images as potential biomarkers to distinguish between CD and UC objectively, quantitatively, and reproducibly.Materials and Methods
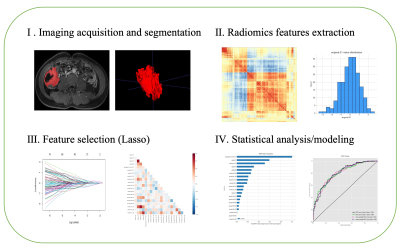
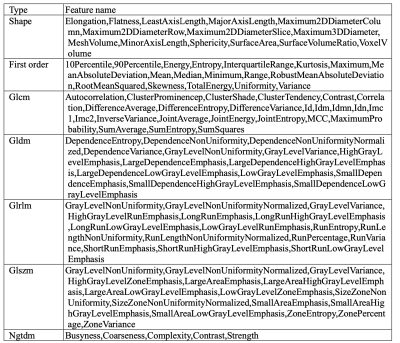
In this retrospective study, a total of 216 patients (mean age 32, SD ± 4.52) underwent MRI scanning, 100 of whom had been previously diagnosed with CD and 116 with UC who underwent colonoscopy and contrast enhanced MRI scans between 2012 and 2021 were included. The protocols of the contrast-enhanced T1-weighted imaging based on mDixon: repetition time = 1100 ms, field of view = 374 × 132 mm2, time echo = 80 ms, 105 axial slices, slice thickness = 1.1 mm, flip angle = 90°. Gadopentetate dimeglumine (0.1 mmol/kg) were taken as the contrast agent. Radiomics features of MRI effectuated were retrospectively analyzed to classify IBD by PyRadiomics4. Imaging was conducted on both 3T MR scanner (Ingenia CX, Philips Healthcare, the Netherlands) and 1.5T MR scanner (Siemens Medical Systems, Erlangen, Germany). Data were extracted by manually drawn regions of interest (ROI) in abnormally thickened intestinal wall. Radiomics feature extraction was carried out in compliance with the image biomarker standardization initiative (IBSI) guidelines. Amount to 1037 radiomics features (for more detailed information, see table 1) were extracted from regions of interest (ROIs) on MRI images after applying two Laplacian-of-Gaussian (LoG) filters known as spatial scaling factors (SSFs) (SSF = 4; SSF = 5) and wavelet transform. Then, calculating the consistency of two expert extraction features, select the features with intra-class correlation coefficient (ICC) >0.75 for subsequent feature screening. Lasso regression model was utilized to pick 15 features and build radiomics signature5. In binary classification, patient labels were determined through previous clinical diagnoses, where all patients had relevant pathological examination reports before MRI scan. Subsequently, multiple machine learning algorithms evaluate the accuracy of these features to classify UC and CD based on scikit-learn6 with python (v3.6.5), together with univariate and multivariate analysis. Within the model, tenfold cross validation was implemented. In addition, supervised learning implementing SHapley Additive exPlanations (SHAP) (https://github.com/slundberg/shap) allowed binary classification of the patients to explain the weight of variables in the models and to ensure their interpretability. Findings were validated through Logistic Regression (LR), K-Nearest Neighbor (KNN), Naïve Bayes (NB), Support Vector Machine (SVM), Gradient Boosting Trees (GBT) and Decision Tree (DT) models with 10-fold cross validation. The proposed analysis workflow is illustrated in Figure 1.Results:
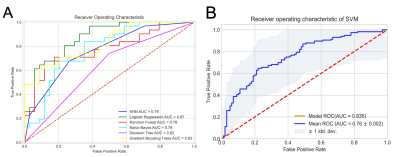
The radiomics signatures were found highly efficient for machine learning to differentiate UC from CD. The highest performance, with an area under the curve (AUC), accuracy, sensitivity, and specificity of 0.874 (95% confidence interval: 0.81–0.91), 80.0, 81.0, and 78.0%, respectively, in the test set to identify the two diseases by Logistic Regression model. The performance of GBT and SVM model is similar. The detailed ROC curves are illustrated in Figure 2.Discussion:
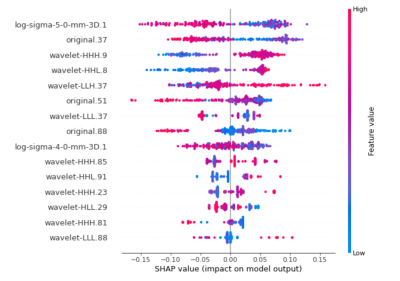
This study investigated the value of CE-MRI radiomics features for differential diagnosis of IBD subtypes. The results show that the features extracted by the imaging features can be used as effective biomarkers for disease differential diagnosis. This is consistent with the previous results7. Radiomics can provide more details of images and help to evaluate the disease more comprehensively and accurately. The study offers some important insights into a deeper understanding of additional information on intestinal thickening and enhancement in IBD. Another observation was that 9 of 15 significant predictors were calculated from decomposed MRI data after the wavelet transform, which are often difficult to judge by the naked eyes of junior radiologists. SHAP summary plot results indicated, among others, “10 Percentile” from log-sigma 5mm 3D, “Contrast” from GLCM, and first-order “Mean” transformed by wavelet as the strongest indicators to predict disease subtype (shown as Figure 3). Above results indicates that wavelet image decomposition enhanced the discriminatory power of radiomics, possibly due to the noise suppression and identification of frequency bands that better capture the actual imaging phenotype variability. Enhanced images can reflect intestinal inflammation more valuable information on blood supply, internal lesions and more.Conclusion:
In our study, the MRI-based radiomics models demonstrated similar performance with the machine learning model in differential diagnosis. Radiomics texture features can serve as potential biomarkers for distinguishing CD and UC. While the radiomics model established by logical regression showed better diagnostic performance than others. The model interpretation of SHAP shows that the characteristics of wavelet features can effectively distinguish the two disease. The radiomics model centered on enhanced MRI shows its robustness in the differential diagnosis of IBD. Radiological features may be a potential source of biomarker-based disease classification and diagnosis.Acknowledgements
No acknowledgement found.References
1. Sands B E. Biomarkers of Inflammation in Inflammatory Bowel Disease[J]. Gastroenterology, 2015, 149(5): 1275-1285.e1272.
2. Biernacka K B, Barańska D, Matera K, et al. The value of magnetic resonance enterography in diagnostic difficulties associated with Crohn's disease[J]. Pol J Radiol, 2021, 86: e143-e150.
3. Maaser C, Sturm A, Vavricka S R, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications[J]. J Crohns Colitis, 2019, 13(2): 144-164.
4. Van Griethuysen J J M, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic Phenotype[J]. Cancer Res, 2017, 77(21): e104-e107.
5. Li Z, Sillanpää M J. Overview of LASSO-related penalized regression methods for quantitative trait mapping and genomic selection[J]. Theor Appl Genet, 2012, 125(3): 419-435.
6. Pedregosa F, Varoquaux G, Gramfort A, et al. Scikit-learn: Machine learning in Python[J]. the Journal of machine Learning research, 2011, 12: 2825-2830.
7. Li H, Mo Y, Huang C, et al. An MSCT-based radiomics nomogram combined with clinical factors can identify Crohn's disease and ulcerative colitis[J]. Ann Transl Med, 2021, 9(7): 572.
Figures