3160
Harmonization of multi-site DTI and NODDI data using the combined association test1Department of Radiology, Graduate School of Medicine, Juntendo University, Tokyo, Japan, 2Center for Evolutionary Cognitive Sciences, Graduate School of Art and Sciences, The University of Tokyo, Tokyo, Japan, 3Department of Radiological Sciences, Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan, 4Department of Radiology, University of Tokyo, Tokyo, Japan, 5Department of Radiology, Toho University Omori Medical Center, Tokyo, Japan
Synopsis
When analyzing multi-site diffusion MRI (dMRI) data, metrics should be harmonized to remove the site effect. In this study, we applied the combined association test (ComBat), which uses regression of covariates with an empirical Bayes framework, for diffusion metrics based on diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). The results showed that ComBat can harmonize site-related effects in DTI and NODDI metrics based on multi-site dMRI while preserving subject biological information, such as sex differences and correlation with age. Thus, ComBat could be applied in large multi-site studies to identify subtle white matter changes.
INTRODUCTION:
Multi-site dMRI data, which increase the statistical power for studying microstructural changes in white matter (WM), include a site effect due to variability in acquisition conditions related to site, scanner, and protocol1, 2. Notably, the site effect in diffusion metrics based on diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI) may be similar to the difference in diffusion metrics related to the condition of interest, e.g., mild traumatic brain injury (mTBI)3, schizophrenia4, and bipolar disorder (BD)5, Autism Spectrum Disorders (ASD). Thus, diffusion metrics calculated using multi-site dMRI must be harmonized to remove this site effect. Here, the combined association test (ComBat)6, which uses regression of covariates with an empirical Bayes, was applied for DTI and NODDI to harmonize site-related difference. Moreover, we confirmed the ability of ComBat to retain biological information unrelated to site effect, including sex difference and correlation with age.METHODS:
Study participantsThe dMRI data were acquired from 69 participants who traveled to 5–6 sites (in total 13 sites, 7 scanners, and 390 scans; Table 1)7. Each dMRI examination was performed using a Siemens 3T scanner with a 32-channel head coil and acquisition parameters shown in Table 2. dMRI data were assigned into five datasets of scan–rescan and four site effect factors (site, scanner, protocol, and site × scanner × protocol).
Diffusion MRI preprocessing
All dMRI data were corrected for susceptibility, eddy-current induced geometric distortions8, and intervolume subject motion9. Intracellular volume fraction (ICVF), isotropic volume fraction (ISOVF), and orientation dispersion index (ODI) were estimated based on the NODDI model using corrected dMRI data10. Fractional anisotropy (FA) and mean diffusivity (MD) were calculated after fitting the diffusion tensor model to the corrected dMRI data with b-values of 0 and 700 (Procedure 2l) or 1500 (Procedure 2) s/mm2 11.
Harmonization of diffusion metrics using ComBat
The average diffusion metrics of each regional WM tract were measured using the ICBM-DTI-81 WM labels atlas12, 13, comprising 25 structures (Figure 1), registered to each subject’s diffusion space. ComBat was applied to the average diffusion metrics of each regional WM tract to harmonize the site effect with age and gender included as biological covariates. The Cohen’s d of the site effect was compared before and after ComBat. To assess whether biological information was retained, the absolute difference of Cohen’s d (|∆d|) between females and males and Spearman’s correlation (|∆rs|) with age were calculated for each diffusion metric. According to Cohen’s guidelines14, 15, Cohen’s d and |∆d| = 0.20, 0.50, 0.80, |∆rs| = 0.10, 0.30, and 0.50 were interpreted as small, medium, or large effect size, respectively.
RESULTS:
Figure 2 (a) and 3 summarize the site effect in the diffusion metrics for each WM tract. The “Protocol” site effect has a large effect size (5.0 ≲ d ≲ 12.0), greatly decreased by ComBat (d ≲ 0.2). As shown in Figure 2 (b), Cohen’s d between females and males and correlation with age were almost unchanged by ComBat (|∆d| ≲ 0.15, |∆rs| ≲ 0.15). However, the variances of biological information in MD were higher than those in the other diffusion metrics (|∆d| ≲ 0.35, |∆rs| ≲ 0.20).DISCUSSION:
This study applied ComBat to harmonize the site effect in DTI and NODDI metrics. Importantly, ComBat retained the biological information, including sex differences and correlation with age, despite the limited participant age range (31.6 ± 9.4 years). Overall, ComBat successfully harmonized the site effect over almost all WM tracts.One limitation is the higher variances in the biological information in MD compared to other diffusion metrics. This may arise because the b-values for fitting DTI and MD are susceptible to the b-value combination16. As a result, MD is greatly harmonized, but the retainability of biological information is relatively lower than that for other diffusion metrics. Thus, attention should be paid to the harmonization results of MD calculated from the difference in b-values.
The ability of ComBat to greatly decrease the effect size of all site-related effects suggests that it could be applied to detect subtle WM changes, e.g., compared to healthy controls, FA of overall WM in schizophrenia (d= –0.42)4; FA of CC, EC, and cingulum in BD (d= –0.43 to –0.54)5; ICVF and ISOVF of CC, EC, and cingulum in mTBI (ICVF: d = –0.31 to 0.42, ISOVF: d= –0.54 to –1.19)3.
Despite the strong harmonization, Cohen’s d between females and males and correlation with age were almost unchanged before and after ComBat. For instance, the correlation in ASD between ICVF in WM and Autism Spectrum Quotient score was negative (r= –0.40 to –0.41)17, the correlation in schizophrenia between FA of CC and cingulum and Positive and Negative Syndrome Scale was negative (r= –0.382 to –0.503)18, and one BD between CC of FA and Life History of Aggression was negative (r = 0.42)19. Thus, the change in biological information due to ComBat was much smaller than that of WM and the correlation in patient populations.
CONCLUSION:
ComBat can harmonize site-related effects in DTI and NODDI metrics based on multi-site dMRI data while preserving subject biological information. Thus, it could be applied to detect subtle WM changes in clinical groups in large multi-site studies.Acknowledgements
This work was supported by research grants from the Program for Brain/MINDS Beyond Program from the Japan Agency for Medical Research andDevelopment (AMED) under Grant Number JP18dm0307024; MEXT-Supported Program for the Private University Research Branding Project;ImPACT Program of Council for Science, Technology and Innovation (Cabinet Oce, Government of Japan); and JSPS KAKENHI Grant NumberJP16K10327.References
1. Andica C, Kamagata K, Hayashi T, et al. Scan-rescan and inter-vendor reproducibility of neurite orientation dispersion and density imaging metrics. Neuroradiology 2020;62:483-494.
2. Pinto MS, Paolella R, Billiet T, et al. Harmonization of Brain Diffusion MRI: Concepts and Methods. Front Neurosci 2020;14:396.
3. Palacios EM, Owen JP, Yuh EL, et al. The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci Adv 2020;6:eaaz6892.
4. Kelly S, Jahanshad N, Zalesky A, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry 2018;23:1261-1269.
5. Favre P, Pauling M, Stout J, et al. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology 2019;44:2285-2293.
6. Fortin JP, Parker D, Tunç B, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017;161:149-170.
7. Koike S, Tanaka SC, Okada T, et al. Brain/MINDS beyond human brain MRI project: A protocol for multi-level harmonization across brain disorders throughout the lifespan. Neuroimage Clin 2021;30:102600.
8. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20:870-888.
9. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 2016;125:1063-1078.
10. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012;61:1000-1016.
11. Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994;103:247-254.
12. Hua K, Zhang J, Wakana S, et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 2008;39:336-347.
13. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007;36:630-644.
14. Cohen J. A power primer. Psychol Bull 1992;112:155-159.
15. Cohen J. Statistical power analysis for the behavioral sciences: Academic press, 2013.
16. Baltzer P, Mann RM, Iima M, et al. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur Radiol 2020;30:1436-1450.
17. Andica C, Kamagata K, Kirino E, et al. Neurite orientation dispersion and density imaging reveals white matter microstructural alterations in adults with autism. Mol Autism 2021;12:48.
18. Lang XE, Zhu D, Zhang G, et al. Sex difference in association of symptoms and white matter deficits in first-episode and drug-naive schizophrenia. Transl Psychiatry 2018;8:281.
19. Saxena K, Tamm L, Walley A, et al. A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. J Child Adolesc Psychopharmacol 2012;22:112-119.
Figures
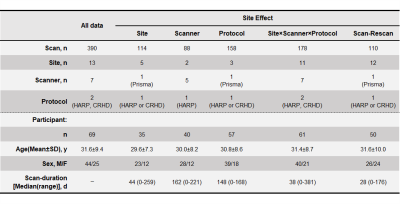
Table 1. Demographic characteristics of study participants.
The dMRI data of 69 participants (13 sites, 7 scanners, a total of 390 scans) was acquired followed by a “hub-and-spoke” design in which participant traveled to 5–6 sites, but not all sites7. dMRI data were assigned into five datasets of scan–rescan and four site effect factors (site, scanner, protocol, and site × scanner × protocol) to evaluate the harmonization performance of ComBat.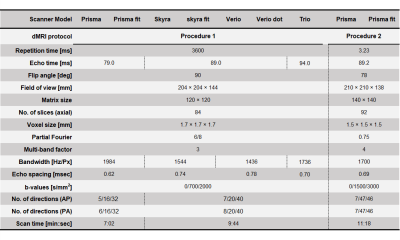
Table 2. Diffusion MRI acquisition parameters.
Each dMRI examination was performed using a Siemens 3T scanner with a 32-channel head coil and the presented acquisition parameters. All dMRI data were corrected for susceptibility, eddy-current induced geometric distortions8, and intervolume subject motion9. NODDI metrics were estimated based on the NODDI model using corrected dMRI data10. DTI metrics were calculated after fitting the diffusion tensor model to the corrected dMRI data with b-values of 0 and 700 ((Procedure 1) or 1500 (Procedure 2) s/mm2 11.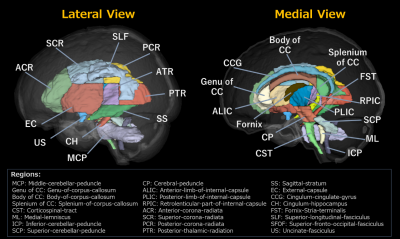
Figure 1. ICBM-DTI-81 WM labels atlas.
The average diffusion metrics of each regional white matter (WM) tract were measured using the ICBM-DTI-81 WM labels atlas15,16, which comprises 25 structures. registered to each participant’s diffusion space.
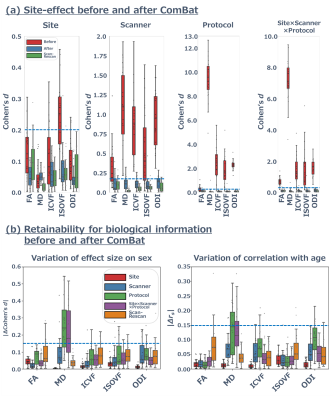
Figure 2. Site effect and retention of biological information before and after applying ComBat.
(a) The site effect of diffusion metrics summarizing each WM tract. Although Cohen’s d of MD with “Protocol” the site effect was notably high (5.0 ≲ d ≲ 12.0), ComBat harmonization greatly decreased Cohen’s d (d ≲ 0.2, blue dotted line) up to Cohen’ d in scan–rescan for all site effect factors and diffusion metrics. (b) Cohen’s d between females and males and correlation with age were almost unchanged before and after applying ComBat (|∆d| ≲ 0.15, |∆rs| ≲ 0.15, blue dotted line).
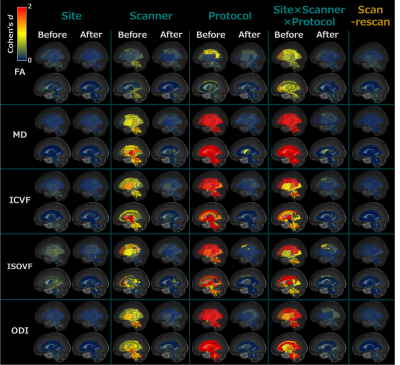
Figure 3. The site effect of diffusion metrics for each WM tract
The rows show Cohen’s d of each diffusion metric in the WM tracts, while the columns show the site effect factor before and after ComBat. The color indicates the effect size of the site effect in the regional WM tracts with blue indicating a low effect size (Cohen’s d of 0), changing to yellow for a moderate effect size (Cohen’s d of 1), and deepening to red for a large effect size (Cohen’s d > 2). Abbreviation: Intracellular volume fraction, ICVF, isotropic volume fraction, ISOVF, and orientation dispersion index, ODI.