3155
Structural connectivity abnormality in trigeminal neuralgia with pain relief using graph theory analysis1Hebei General Hospital, Shijiazhuang, China, 2GE Healthcare, Shijiazhuang, China
Synopsis
Trigeminal neuralgia (TN) characterized by recurring paroxysmal pain, severely affects the quality of life (QoL) of patients. In this study, we compared brain network frame remodeling in TN patients before and after percutaneous micro-balloon compression (PMC) operation with healthy controls (HCs) using graph theory analysis. Both TN pre-treatment patients (Pre) and HCs exhibited small-world network organization. Pre TN patients had abnormal global network construction, Pos recovered, but in local network index increased. Graph theory analysis could be a useful method to detect instant change after operation.
introduction
TN is a chronic neuropathic pain disorder, characterized by recurring paroxysmal, electric shock-like pain along the trigeminal nerve branches(1). TN may increase the risk of newly diagnosed mental illness, such as depression, anxiety, and sleep disorders(2-4), and subsequently can severely affects the quality of life (QoL) of patients. In recent years, many studies identified that both functional and structural brain abnormalities may represent biomarker for TN(5, 6). Graph theory (GT) analysis or connectome analysis have rapidly found application in the clinical neurosciences for explain imbalances between integration and segregation after brain disruption(7). GT supplies us with an image expression to these complex brain networks and might illustrate functional connectivity in local and global networks. However, there are so far few studies apply GT in TN even following operations. We hypothesized that effective treatment would be associated with the remodeling of brain abnormalities.Material and Methods
We enrolled 23 TN patients treated with PMC at our Hospital between October 2019 and June 2020 and enrolled matched healthy control subjects for age, sex, and level of education. The inclusion of patients with idiopathic TN had pre -treatment MRI scans within 2 to 4 days, post-treatment MRI scan was taken between 24h and 36h after surgery in case of the effect of anesthetic sevoflurane. All healthy controls only receive one time scan. Clinical characteristics and outcome assessment of TN: sex, age, education level, disease duration of TN, affected side, presence of neurovascular compression, visual analog scale (VAS) before and after the operation. Disease duration of TN was defined as the amount of time from diagnosing TN for the first time to scanning before the operation. All participants are right-handed. Brain imaging data were acquired on GE 3.0T MRI scanner (General Electric Medical System, Discovery MR750w) with a standard 8-channel head coil. High - resolution whole-brain T1-weighted images were acquired using a 3D-T1 BRAVO sequence with following parameters: TR = 8.5 ms, TE = 3.2 ms, matrix = 256 × 256, flip angle = 13°, acquisition time = 3minutes 15 seconds. Finally, resting-state fMRI blood-oxygen level dependent (BOLD) imaging was carried out with the instruction to close eyes, not think about anything else and not sleep, and with the following parameters: TR = 2000 ms, TE = 30 ms, FOV= 24cm × 24cm, matrix = 64 × 64,flip angle = 90°, acquisition time= 6 minutes 40 seconds, 200 time points. The comparison of functional connectivity between HCs and TN patients was carried out using one-way independent analysis of covariance (ANCOVA) and two-sample T-tests. Comparisons longitudinal samples within the patient group before and after surgery were carried out using paired t-test. False discovery rate (FDR) correction was used for multiple comparisons.Results
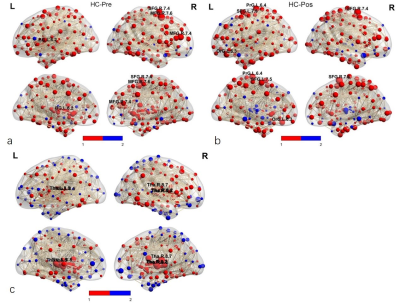
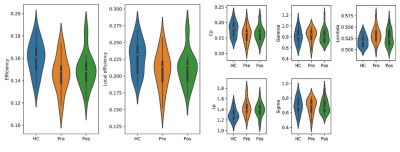
One patient was refused to take second scan for failing operation, 22 patients (10 females and 12 males, average age 59.3 ± 5.3 years) were success for pain relief and took second scans. There were no significant differences in age, sex, or education level between the TN patients and HCs (P > 0.05). The brain structural networks in both HCs and Pre TN patients exhibited small-world properties. Compared with the HC, Pre TN patients were found to have significantly decreased global efficiency and higher values in the shortest path length (P < 0.05, FDR corrected), but Pos TN patients were found no difference. No significant differences were found in local efficiency and SW (P > 0.05) between the two groups. (Fig.1). ANCOVA analysis showed significant differences in nodal efficiency (P < 0.005, FDR corrected) . In between-group comparison, Pre TN patients showed decreased nodal efficiency in the right SFG 7.4, MFG 7.6, MFG 7.4, and left globus pallidus basal ganglia (BG 6.2) compared with HCs (P < 0.005, FDR corrected). Pos TN patients showed a decreased nodal efficiency in right SFG 7.4, left precentral gyrus trunk region (PrG 6.4), medial superior frontal gyrus (SFG 7.5), and lateral orbital gyrus (OrG 6.3) compared with HCs (P < 0.005, FDR corrected). Moreover, Pos TN patients showed an increased nodal efficiency in the bilateral thalamus (right pre-motor thalamus, caudal temporal thalamus, lateral pre-frontal thalamus, left posterior parietal thalamus, and lateral pre-frontal thalamus) compared with HCs (Fig. 2). Edges identified in HC_Pos TN is 298, half of HC_Pre (526). While 18 edges were identified between Pos TN and Pre TN patients, of which 16 edges were increased in Pos TN patients and 2 were reduced compared with Pre TN patients.Discussion and Conclusion
We demonstrated GTA network topological organization in addressing functional plasticity in the brain of TN patients, with pain relief operations less than two days earlier. We found that Pre TN patients exhibited SW properties. Global efficiency in Pre TN patients decreased, but in Pos TN patients recovered. Moreover, compared with Pre TN patients, Pos TN patients had a significantly higher node efficiency and enhanced connectivity in the thalamus, in particular in the right thalamus. The parietal lobe and the ACC exhibited a negatively connection distance in Pos TN patients compared with Pre. Our results may advance the knowledge of how topological organization changes after neurosurgical treatment of TN.Acknowledgements
No acknowledgement found.References
1. Nurmikko TE, PR. Trigeminal neuralgiaÐpathophysiology, diagnosis and current treatment. British Journal of Anaesthesia 2001;87(1):117-32.
2. Di Stefano G, Maarbjerg S, Nurmikko T, Truini A, Cruccu G. Triggering trigeminal neuralgia. Cephalalgia 2017;0:1-8.
3. Gambeta EC, JG; Zamponi, GW. Trigeminal Neuralgia: an overview from pathophysiology to pharmacological treatments. Molecular Pain 2019;16:1744806920901890.
4. Janet M. Torpy M. Trigeminal Neuralgia. The Journal of the American Medical Association 2013;309:1058.
5. Hayes DJ, Chen DQ, Zhong J, et al. Affective Circuitry Alterations in Patients with Trigeminal Neuralgia. Front Neuroanat 2017;11:73.
6. Seminowicz DW, TH; Naso, L; Hatami-Khoroushahi, Z; Fallatah, S; Ware, MA; Jarzem, P; Bushnell, MC; Shir, Y; Ouellet, JA; Stone, LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci 2011;31:7540-7550.
7. Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheum 2010;62:2930-2940.
8. Tomasi D, Volkow ND. Association Between Brain Activation and Functional Connectivity. Cereb Cortex 2019;29:1984-1996.
Figures