3153
Dynamic functional network connectivity and its association with lipid metabolism in Alzheimer’s disease1Southeast University, Nanjing, China, 2Southeast University Affiliated ZhongDa Hospital, Nanjing, China
Synopsis
Brain networks of Alzheimer’s disease (AD) represent the fluctuating functional connectivity with time. Using the sliding-window method, this study identified two different functional connectivity states at the large-scale network level in AD spectrum. The AD patients took more time in the tense-connected state II than the non-AD populations. Moreover, lipid metabolism-related factors affected the dynamic network connectivity across the AD spectrum populations. These results provide insights into the neural biological underpinnings of the dynamic networks reorganization for AD pathophysiology.
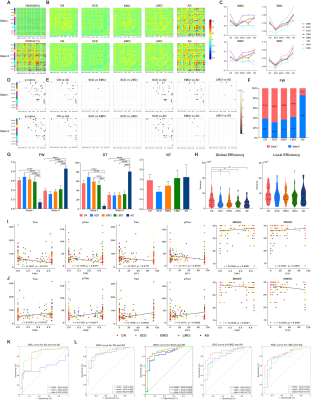
METHODS: We included 242 subjects, including 181 subjects with AD spectrum and 61 cognitively normal subjects. The data of lipid metabolism-related genes, serum lipid metabolites, cerebrospinal fluid (CSF) core biomarkers, structural and resting-state functional MRI, and cognitive assessment were obtained for all subjects. We used sliding-window method and k-means algorithm to obtain the dFNC matrix of all subjects and conducted cluster analysis. Spearman correlation analysis was employed to analyze the associations of dFNC temporal properties to CSF core biomarkers, cognitive scores and lipid metabolites. Polygenic score (PGS) were constructed based on the odds ratio (OR) value of each gene locus, and the lipid composite score was constructed based on the significant correlative lipid components. We also tested dynamic network difference and dFNC associations with CSF and behavioral phenotyping in lipid-related subgroups. To explore the casual relationship among lipid-related indicators, dFNC, CSF pathology and behavioral phenotype, we applied the mediation effect analysis. Finally, the abnormal dFNC was used as an objective diagnostic biomarker to classify the AD spectrum populations by support vector machine (SVM) model.
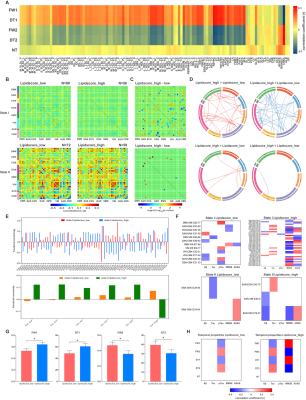
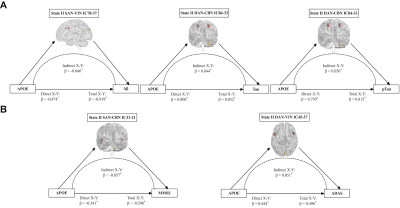
RESULTS: Two stable reoccurring connectivity states were identified: frequent but weak-connected state I, and less frequent but strong-connected state II. The AD patients showed strong connectivity strength in both states. They represented significantly differential connectivity and temporal properties compared to non-AD participants in each state. The SVM analysis showed that the dFNC, especially state I connectivity, were better in differentiating AD from other groups of subjects. Interestingly, they altered brain preference to dynamic connectivity state, in that they tended to dwell longer in the densely connectivity state II, whereas the non-AD populations were prone to reside in the sparsely state I. Then, the temporal properties of two states, including fractional windows and mean dwell time, were anti-correlated with CSF core biomarkers, cognitive scores, and lipid metabolites. More importantly, lipid-related subgroup analysis found that the effect of lipid composite score over dynamic connectivity differences was mainly in state I, whereas APOE ε4 showed a wide effect on state II network differences. The effects of PGS on the two states were not significantly different, but when APOE was removed, the effect of polygenes on state I network differences were more involved in within-network connectivity. In addition, the state II differential connections between APOE ε4 genotype subgroups mediates the effects of APOE genotype on CSF and cognitive phenotypes.
DISCUSSION: Subjects spent more time in weakly-connected state I, where new connections were more easily established to promote higher network flexibility 1. However, less frequent state II with strong connectivity might compensate for the loss of intertwined brain function under network destruction. Herein, AD patients reversed the brain’s preference for state and took a long time with higher frequency in state II. Contrary to popular belief 2, the connectivity strength of AD patients reached the highest point at both states, probably because they were still at the early stages of AD and had restricted compensatory capacity. There was a significant group-level network difference between association cortex system and sensory cortex system, similar to previous findings 3, 4. Network disruption between the two systems might characterize biological and behavioral phenotyping in AD. Herein, temporal variability was lower in AD than in healthy subjects, consistent with previous studies 4, 5.Moreover, the temporal properties and molecular, clinical phenotyping had an anti-correlation pattern between the two states. For instance, frequent occurrences and staying longer in state I had a protective role, thus alleviating tau pathology and improving cognition, while state II showed the opposite effect (destructive effect), causing the biological and behavioral underpinnings due to dynamic temporal characterization.The SVM analysis also showed that dFNC had better predictive power than sFNC in distinguishing AD and non-AD groups, suggesting the essentiality of extracting distinct dynamic states from the conventional sFNC. The different lipid architectures mainly influenced dFNC in state I, while the APOE ε4 mainly impacted dFNC in state II, suggesting that lipid profile and APOE have different impacts on dFNC. The PGS showed more within-network connectivity in state I after removing APOE ε4, indicating that APOE ε4 guided the direction of polygenes to destroy between-network connectivity. The differential state II connections had an indirect effect on the relationship between APOE and CSF, cognitive indicators. The long-range connections of salience and dorsal attention network interacted with visual and cerebellum network are probably related to top-down attentional control.
CONCLUSION: This study is the first to explore the temporal-spatial patterns of the whole brain dynamic network connectivity and its temporal properties across the entire cognitive spectrum ranging from normal cognition to AD, and reveals the effect of lipid biological factors over the dynamic large-scale brain networks, providing a new insight into the lipid biological basis of the reorganization of dynamic brain networks for AD.
Acknowledgements
This study was granted funding by the National Key Projects for Research and Development Program of China (2016YFC1305800,2016YFC1305802, CMX), the National Natural Science Foundation of China (82071204,81871069, CMX), the Key Project for Research and Development Program of Jiangsu Province (BE2018741), the Nanjing International Joint Research and Development Project (201715013), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_0115).
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904), DOD ADNI (Department of Defense award number W81XWH-12-2-0012), and the Alzheimer's Disease Metabolomics Consortium (National Institute on Aging R01AG046171, RF1AG051550 and 3U01AG024904-09S4). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.References
1. Bonkhoff AK, Espinoza FA, Gazula H, et al. Acute ischaemic stroke alters the brain's preference for distinct dynamic connectivity states. Brain : a journal of neurology. May 1 2020;143(5):1525-1540.
2. Jones DT, Knopman DS, Gunter JL, et al. Cascading network failure across the Alzheimer's disease spectrum. Brain : a journal of neurology. Feb 2016;139(Pt 2):547-62.
3. Fu Z, Caprihan A, Chen J, et al. Altered static and dynamic functional network connectivity in Alzheimer's disease and subcortical ischemic vascular disease: shared and specific brain connectivity abnormalities. Human brain mapping. Aug 1 2019;40(11):3203-3221.
4. Gu Y, Lin Y, Huang L, et al. Abnormal dynamic functional connectivity in Alzheimer's disease. CNS neuroscience & therapeutics. Sep 2020;26(9):962-971.
5. Schumacher J, Peraza LR, Firbank M, et al. Dynamic functional connectivity changes in dementia with Lewy bodies and Alzheimer's disease. NeuroImage Clinical. 2019;22:101812.Figures